Introduction
Rocky shores are naturally disturbed environments where secondary and tertiary successions occur due, notably, to direct and indirect action of waves removing biota (Hawkins et al., 2020). Patchiness is thus a fundamental feature of most rocky shores where macrobenthic communities correspond to mosaics of species assemblages on various spatial scales in different succession stages, from cleared rock to complete cover by a dominant species (Raffaelli & Hawkins, 1999). Primary succession, the colonization of virgin substrata, can also be initiated on rocky shores, either naturally by flaking of the rock or artificially by the placement of human-made structures. The early physicochemical events occurring over very short time periods allow the rapid colonization of bare substrata by bacteria and diatoms. After this microbiotic phase, the macrobiotic succession on rocky shores typically involves a transition from a suite of opportunistic and ephemeral species which display minimal variation in functional traits, to a more functionally diverse mature, more or less stable, community including slow-growing perennial species (Noël et al., 2009). This general pattern and the mechanisms implied in the replacement of species are influenced by a number of physical and biological factors. Macrobiotic succession on rocky shores is thus highly variable and context dependent. Furthermore, on most rocky shores, it is likely that succession never stops with small-scale disturbances opening up resources all the time (Raffaelli & Hawkins, 1999) and the perception of the establishment of a stable community is largely temporal scale dependent (Jenkins & Uya, 2016).
On sheltered and semi-exposed intertidal rocky shores, the mature community is often characterized by canopy-forming brown algae. Canopy-forming algae are foundation species that play a pivotal role by mitigating stressful abiotic conditions (Bulleri, 2009) and constitute highly productive systems (Mann, 1973). Loss or reduction of canopy-forming algae, as a consequence of local and global stressors, have direct and indirect effects on benthic communities, reflected in diversity and abundance reductions, shifts in composition and ecosystem functioning (Mineur et al., 2015). There is thus a clear need to understand the mechanisms that control their establishment and persistence. Succession has been the focus of intensive research on rocky shores (Hawkins et al., 2020 and references therein) but few studies have explicitly measured changes in community primary production through succession from opportunistic ephemerals to dense canopy. A species-specific productivity decrease with increasing successional status is predictable but differences in the productivity of component species, measured per unit biomass, do not necessarily scale to the productivity of the community as a whole (Noël et al., 2009).
The present study associates long-term macrobenthic community primary production measurements and community structure assessments over succession initiated experimentally by placing bare substrata in a semi-exposed intertidal rocky shore. The experiment was conducted in two communities adjacent over the emersion gradient, the mid and low-mid intertidal communities dominated by canopy-forming algae, Fucus vesiculosus and Fucus serratus respectively. These communities consist of dense and complex assemblages composed of Fucus spp with associated epibiont and understory algae and invertebrates. Both communities exhibit high metabolic activity, gross community primary production reaching 1 gC m-2 h-1 during spring and summer midday emersions (Bordeyne et al., 2015). Experimental bare substrata units consisted on slabs built with materials of the same nature of the rock and which surface was made rough as surrogates of the natural habitat. Those slabs were spread out in winter in the established communities to be influenced by the same local environmental variation and supply of propagules. With this survey, we intended to measure the functional effect of changes in the community structure through succession and to compare the timing of successional sequences at two levels of the emersion gradient in a rocky shore system. We hypothesized that, due to hasher environmental conditions, the timing of successional sequences would be slower in the mid intertidal than in the low-mid intertidal.
Materials and Methods
Study site and experiment set up
This experiment was conducted on an intertidal boulder reef (Karreg Ar Vraz) located in front of the Roscoff Marine Station (48°43.754’N, 3°59.420’W) in the southwestern part of the English Channel. This semi-exposed shore is subjected to a semi-diurnal tidal cycle, with a maximal range of about 9 m. It presents a typical vertical distribution of communities dominated by canopy-forming brown algae. The mid-intertidal level (3.0 to 4.0 m above chart datum) is characterized by a Fucus vesiculosus community and the low mid-intertidal level (2.5 to 3.0 m above chart datum) is characterized by a F. serratus community. At the mid-intertidal level, F. vesiculosus density is about 45 ind m-2, accounting for 2 to 5 kgFW m-2 according to the season (Bordeyne, 2016). At the low mid-intertidal level, F. serratus density is about 70 ind m-2 accounting for 2 to 12 kgFW m-2 (Bordeyne, 2016). Both communities are known to exhibit high metabolic activity year-round: gross primary production in the range 400-1000 mg C m-2 h-1 and respiration in the range 100-500 mg C m-2 h-1, when measured at the beginning of emersion period (Bordeyne et al., 2015).
In February 2013, bare granite slabs (0.4 × 0.4 m) with rough surface were haphazardly attached (as flat as possible) to large boulders of the mid-intertidal level in the F. vesiculosus area and of the low mid-intertidal level in the F. serratus area of the Karreg Ar Vraz site. Slabs (21 in the F. vesiculosus area, 20 in the F. serratus area) were arranged to be 1 to 3 m away one from each other within areas, and were separated by a distance of approx. 30 m between areas.
Macrobenthic community metabolism
The metabolism of settled communities was assessed from March 2013 to September 2019 (i.e. from one month to 78 months, 6.5 years, after the setup of slabs), with a delay between two sampling dates varying from 1 to 7 months (leading to a total of 25 sampling dates). At each sampling date, metabolism of settled communities was assessed on 3 to 7 randomly chosen slabs in each area. Metabolism was assessed by measuring carbon dioxide (CO2) fluxes at the air-slab interface inside benthic chambers, at the onset of emersion period of spring tides (around midday). This period has previously been shown to be the most favourable for primary production of such intertidal fucoid stands (Migné et al., 2021). Each benthic chamber, which is made of a transparent Perspex® dome with a transparent air-tight Perspex® base, has a covering surface of 0.3 x 0.3 m and a total volume of 17.7 or 26.5 L (depending on the amount of Fucus settled on the slabs). It was sealed to the slab (using bungees) and was connected to an infrared CO2 gas analyzer (LiCor Li-820) in a closed air circuit (with a flow of about 1 L min-1). CO2 air concentration (μmolCO2 molair-1) was recorded every 15 s for 5 to 15 min during incubation to calculate CO2 fluxes as described in Migné et al. (2002). Net community production (NCP) and community respiration (CR) were examined successively in ambient light and in darkness (by covering the chamber), respectively. Photosynthetically available radiations (PAR, in µmol of photons m-2 s-1) were measured close to the incubation chambers, using a planar sensor (Li-Cor QuantumSA-190). PAR were recorded every minute to ensure that measurements in ambient light were performed under saturating irradiance. That is PAR levels above the onset of light saturation which was previously determined to vary seasonally between 250 and 800 µmol m-2 s-1 for the F. vesiculosus community and between 200 and 500 µmol m-2 s-1 for the F. serratus community (Bordeyne, 2016). Benthic chambers were opened between two consecutive incubations to renew the ambient air. Gross community production (GCP) was calculated as the sum of NCP and CR. Metabolic rates were calculated according to the covering surface of incubation chambers (i.e. 0.09 m²) and expressed in carbon units (mg C m-2 h-1) assuming a molar volume of 22.4 L mol-1 at standard temperature and pressure and a molar mass of 12 g C molCO2-1.
Macrobenthic community structure
The survey of the structure of settled communities started as far as some settled Fucus were identifiable to the species level, that was in September 2013 (i.e. seven months after the setup of slabs) and continued until March 2023 (i.e. 121 months, 10 years, after the setup of slabs). Non-destructive sampling of biota colonising the slabs was conducted during low tide, every 3 months until September 2016 and then every 6 months (leading to a total of 25 sampling dates in each area). Algae and invertebrates were identified in the field to the lowest possible taxonomic level, being usually the species level but genus, family, or order level for some colonial invertebrates (e.g. bryozoans, ascidians) and morphologically similar taxa (e.g. ectocarpales, amphipods). Density was determined for Fucus identifiable to the species level and for countable invertebrates (e.g. gastropod species). Identification and count were done on all available slabs (i.e. not detached and returned during the survey) in the F. vesiculosus area (21 slabs until March 2017 and 17 to 14 slabs from September 2017 to March 2023), on all available slabs in the F. serratus area (20 slabs until June 2015 and 19 to 15 slabs from March 2018 to March 2023) or on 9 to 12 haphazardly chosen slabs at the period of the highest colonisation by Fucus (from September 2015 to September 2017).
Before any data analysis, the sampling effort was homogenised by randomly selecting 9 slabs among the slabs on which the structure of the macrobenthic community was assessed at each sampling date, in each area. Given the small size of the slabs and the high small spatial scale variability on rocky shores, data were pooled for the 9 slabs (representing a sampling surface of 1.44 m2) to better highlight the temporal variability of diversity metrics in each area. Shannon diversity and Pielou evenness were calculated based on abundance data of countable invertebrates obtained after pooling the 9 slabs at each sampling date in each area. Pooling the data for the 9 slabs also allowed to calculate the occurrence of each taxa (as the number of slabs bearing the taxa among the 9 slabs) and to analyse similarities between sampling dates and areas based on quantitative data for all the observed taxa. Bray-Curtis similarity was calculated between every pair of samples (in a 72 taxa x 50 samples matrix using the software PRIMER). Samples were then grouped by hierarchical clustering (group-average link) based on the Bray-Curtis similarities to explore spatio-temporal structuring.
Results
Macrobenthic community metabolism
Metabolism of the macrobenthic communities settled on the slabs over the 6.5-year survey showed the same global temporal trend at the two intertidal levels, reaching higher rates in the lower level (i.e. in the Fucus serratus area, Figure 1).
Mean (± se) gross community production (GCP) measured on slabs set at mid-intertidal level in the F. vesiculosus area ranged from 15.53 ± 5.72 to 428.58 ± 171.35 mg C m-2 h-1. The minimum and the maximum were observed 5 and 54 months after the slabs setting respectively (Figure 1A). Mean GCP measured on slabs set at low mid-intertidal level in the F. serratus area ranged from 10.60 ± 8.70 to 839.44 ± 309.13 mg C m-2 h-1. The minimum and the maximum were observed 3 and 27 months after the slabs setting respectively (Figure 1A).
Mean (± se) community respiration (CR) measured on slabs set at mid-intertidal level in the F. vesiculosus area ranged from 5.80 ± 0.92 to 311.08 ± 157.76 mg C m-2 h-1. The minimum and the maximum were observed 6 and 30 months after the slabs setting respectively (Figure 1B). Mean CR measured on slabs set at low mid-intertidal level in the F. serratus area ranged from 1.25 ± 0.75 to 761.02 ± 459.58 mg C m-2 h-1. The minimum and the maximum were observed 10 and 43 months after the slabs setting respectively (Figure 1B).
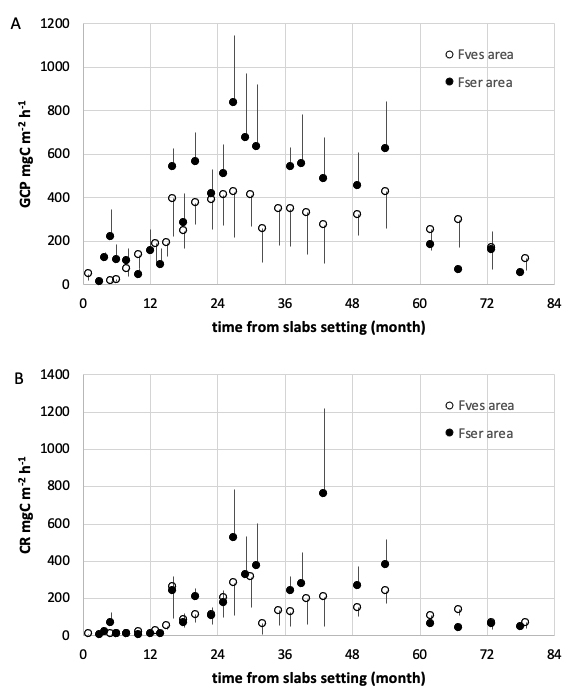
Figure 1 - Mean gross community primary production (GCP, A) and community respiration (CR, B) measured over time on slabs set in the Fucus vesiculosus (Fves) and Fucus serratus (Fser) areas. Error bars indicate standard errors (3 ≤ n ≤ 7).
Macrobenthic community structure
First Fucus individuals settled on slabs that were identifiable to the species level were Fucus vesiculosus individuals in both areas (7 and 10 months after the slabs setting in the F. vesiculosus and F. serratus area respectively). Their density increased rapidly to reach a mean (± se) of 13 ± 5 ind/slab (28 months after the slabs setting) in the F. vesiculosus area (Figure 2A) and of 15 ± 4 ind/slab (16 months after the slabs setting) in the F. serratus area (Figure 2.B.). The F. vesiculosus density then decreased in both areas with mean less than 1 ind/slab since March 2019 (72 months after the slabs setting) in the F. vesiculosus area (Figure 2A) and since March 2018 (61 months after the slabs setting) in the F. serratus area (Figure 2B).
Few Fucus serratus individuals settled on slabs set in the F. vesiculosus area (with a mean in the range 0.1-1.6 ind/slab during the period 37-66 months after the slabs setting, Figure 2A). F. serratus individuals were identifiable on slabs set in the F. serratus area 16 months after the slabs setting, at a mean density of 3 ± 2 ind/slab (Figure 2B). Their mean density increased to 7 ± 3 ind/slab 37 months after the slabs setting and then decreased to less than 1 ind/slab 66 months after the slabs setting (Figure 2B).
At the beginning of the survey, the Patella sp. density increased concurrently with the F. vesiculosus density on slabs in the F. vesiculosus area and with F. serratus density on slabs in the F. serratus area. The Patella sp. density continued to increase when Fucus densities decreased and its mean reached a maximum of 16 ind/slab (85 months after the slabs setting) in the F. vesiculosus area (Figure 2A) and of 20 ind/slab (79 months after the slabs setting) in the F. serratus area (Figure 2B). The Patella sp. density decreased at the end of the survey and, 121 months after the slabs setting, its mean was 5 ind/slab in the F. vesiculosus area (Figure 2A) and 15 ind/slab in the F. serratus area (Figure 2B).
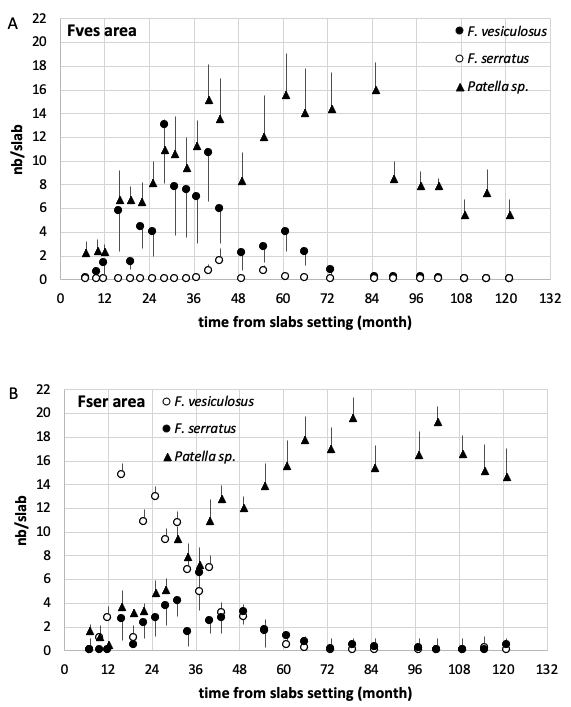
Figure 2 - Mean number of individuals of Fucus vesiculosus, Fucus serratus and Patella sp. observed over time per slab (0.4 x 0.4 m) set in the F. vesiculosus (Fves, A) and F. serratus (Fser, B) areas. Error bars indicate standard errors (n = 9).
Mean (± se) taxa richness per slab (0.4 x 0.4 m), including macroalgae and invertebrates, varied over the survey in the range 3.4 ± 0.4 - 16.4 ± 3.3 in the F. vesiculosus area and in the range 5.4 ± 1.0 - 21.9 ± 3.7 in the F. serratus area. The maximum was observed in June 2016 (40 months after the slabs setting) in both areas. On the 9 pooled slabs (i.e. sampling surface of 1.44 m2), total taxa richness (Stotal) varied in the range 6 - 39 in the F. vesiculosus area and in the range 7 - 51 in the F. serratus area, with a maximum observed in June and September 2016 (40 and 43 months after the slabs setting) in the F. vesiculosus area (Figure 3A) and in September 2016 in the F. serratus area (Figure 3C). Richness in countable invertebrates (Scount) varied in the range 3 - 15 in the F. vesiculosus area (Figure 3A) and in the range 5 - 19 in the F. serratus area (Figure 3C), with a maximum observed in June 2016 in both areas. Shannon diversity (H’) and Pielou’s evenness (J), calculated based on countable invertebrate taxa on the 9 pooled slabs, tended to decrease over the survey in the F. vesiculosus area with maximum values of 2.54 and 0.81 observed 10 and 7 months after the slabs setting, respectively, and minimum values of 0.73 and 0.37 observed 109 months after the slabs setting (Figure 3B). In the F. serratus area, H’ remained higher than 2.00 until September 2017 then decreased to values around 1.00 since March 2020 while J decreased over the survey with a maximum value of 0.82 observed 12 months after the slabs setting and a minimum value of 0.27 observed 102 months after the slabs setting (Figure 3D).
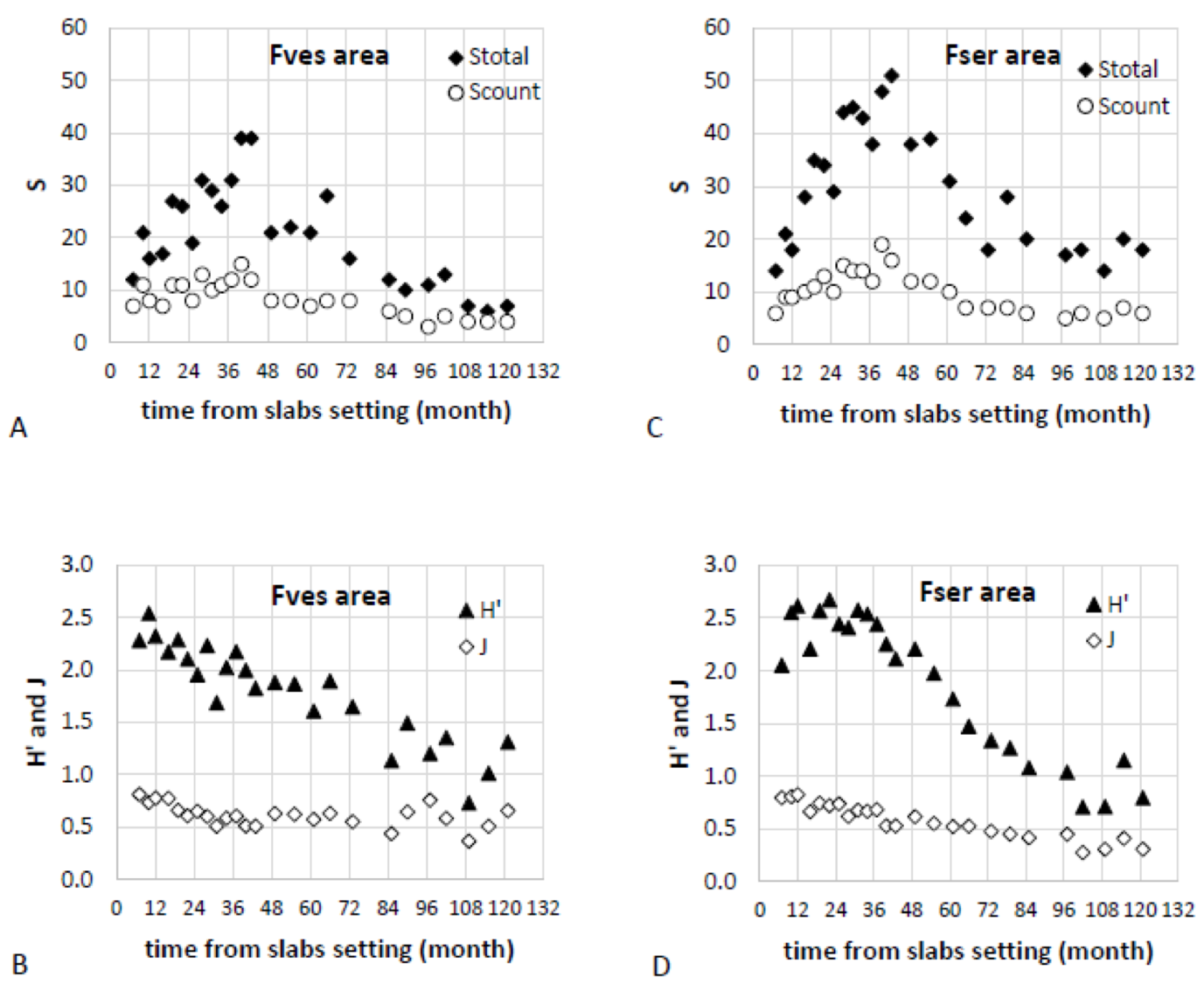
Figure 3 - Total number of taxa (Stotal) and number of countable invertebrate taxa (Scount) on the 9 pooled slabs (1.44 m2), Shannon diversity index (H’) and Pielou’s evenness (J) calculated based on countable invertebrate taxa on the 9 pooled slabs observed over time in the Fucus vesiculosus (Fves, A and B) and Fucus serratus (Fser, C and D) areas.
A total of 72 taxa were identified along the survey, including 27 algae and 45 invertebrates, most being observed in both areas (23 algae and 31 invertebrates). Among taxa commonly observed but only in one area, there were the barnacle Austrominius modestus in the F. vesiculosus area and the brown alga Ascophyllum nodosum, the gastropod Calliostoma zizyphinum and the bryozoan Flustrellidra hispida in the F. serratus area. The hierarchical clustering based on Bray-Curtis similarity between occurrence of taxa on slabs through time showed five groups of samples (Figure 4). Four groups were formed by samples performed in the same area during a certain period: from 16 to 73 and from 85 to 121 months after the slabs setting (grouped, respectively, at a 57 and 72% similarity level) in the F. vesiculosus area and from 16 to 49 and from 55 to 121 months after the slabs setting (grouped, respectively, at a 65 and 69% similarity level) in the F. serratus area. The fifth group were formed (at a 53% similarity level) by the samples performed during the first year of the survey in both areas.
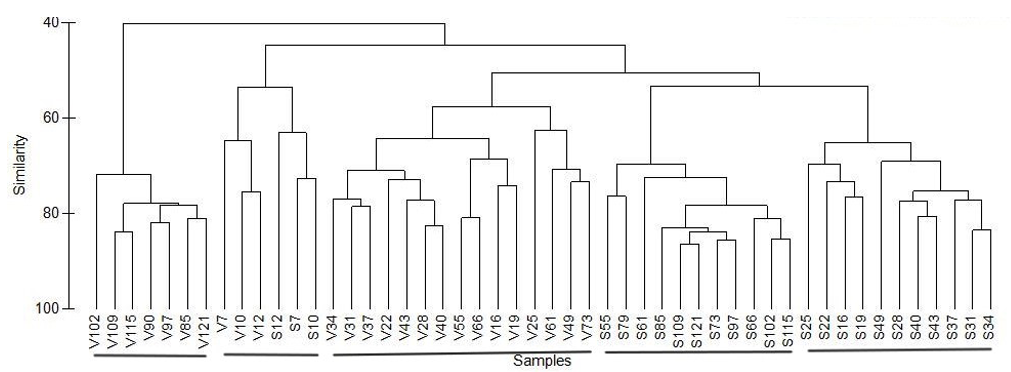
Figure 4 - Dendrogram obtained by hierarchical clustering (average linkage) based on Bray-Curtis similarity between occurrence of taxa on 9 slabs through time (from 7 to 121 months after setting) in the Fucus vesiculosus (V7-V121) and Fucus serratus (S7-S121) areas.
Discussion
Primary succession at two tidal levels of a rocky shore
The present 10-year survey of the structure of communities established on bare substrata at two tidal levels on a rocky shore showed three distinct periods. The first period corresponded to the first year following the availability of bare substrata at both tidal levels. The second period was longer at the higher tidal level, about 6 years in the Fucus vesiculosus area at mid-intertidal versus 4 years in the Fucus serratus area at low mid-intertidal level. The third period corresponded to the last years of the survey: from about the seventh and fifth year after bare substrata was available at mid-intertidal and low mid-intertidal level respectively. While of different duration, these three periods presented similar characteristics at the two tidal levels (Figure 5). During the first period, communities with a low density of Fucus (averaging 6 ind m-2) and a rather low richness of taxa (about 17 taxa observed in an area of 1.44 m2) exhibited low metabolic activity (gross primary production about 100 mg C m-2 h-1 and respiration about 10 mg C m-2 h-1). During the second period, communities with a high density of Fucus (averaging 35 and 67 ind m-2 in the F. vesiculosus and the F. serratus area respectively) and a rather high richness of taxa (about 26 and 39 taxa in the F. vesiculosus and the F. serratus area respectively) exhibited high metabolic activity (gross primary production about 350 and 550 mg C m-2 h-1 and respiration about 150 and 300 mg C m-2 h-1 in the F. vesiculosus and the F. serratus area respectively). During the third period, communities with a low density of Fucus (less than 5 ind m-2) and a rather low richness of taxa (about 9 and 22 taxa in the F. vesiculosus and the F. serratus area respectively) exhibited low metabolic activity (gross primary production about 100 mg C m-2 h-1 and respiration about 50 mg C m-2 h-1 in the F. serratus area, no data for the F. vesiculosus area). During the second period, that is after recruitment and growth of Fucus, the structure and metabolism of the communities set on the slabs were then similar to the structure and metabolism of the communities established at each tidal level (Bordeyne, 2016; Bordeyne et al., 2015). The transition between the second and the third period was marked by the loss of Fucus individuals. The loss of the foundation species (either F. vesiculosus or F. serratus) was accompanied by a decline in taxa richness as well as a decline in diversity of mobile invertebrates (Figure 5). During the first and the second periods, the dominant mobile invertebrate, accounting for about 30% of the total abundance of counted invertebrates, was a top shell or a periwinkle (Phorcus lineatus and Steromphala umbilicalis in the F. vesiculosus area the first and second period respectively; Steromphala pennanti and Littorina obtusata in the F. serratus area the first and second period respectively). During the third period, the dominant mobile invertebrate was a limpet (Patella sp.) accounting for about 75% of the total abundance of counted invertebrates in both areas.
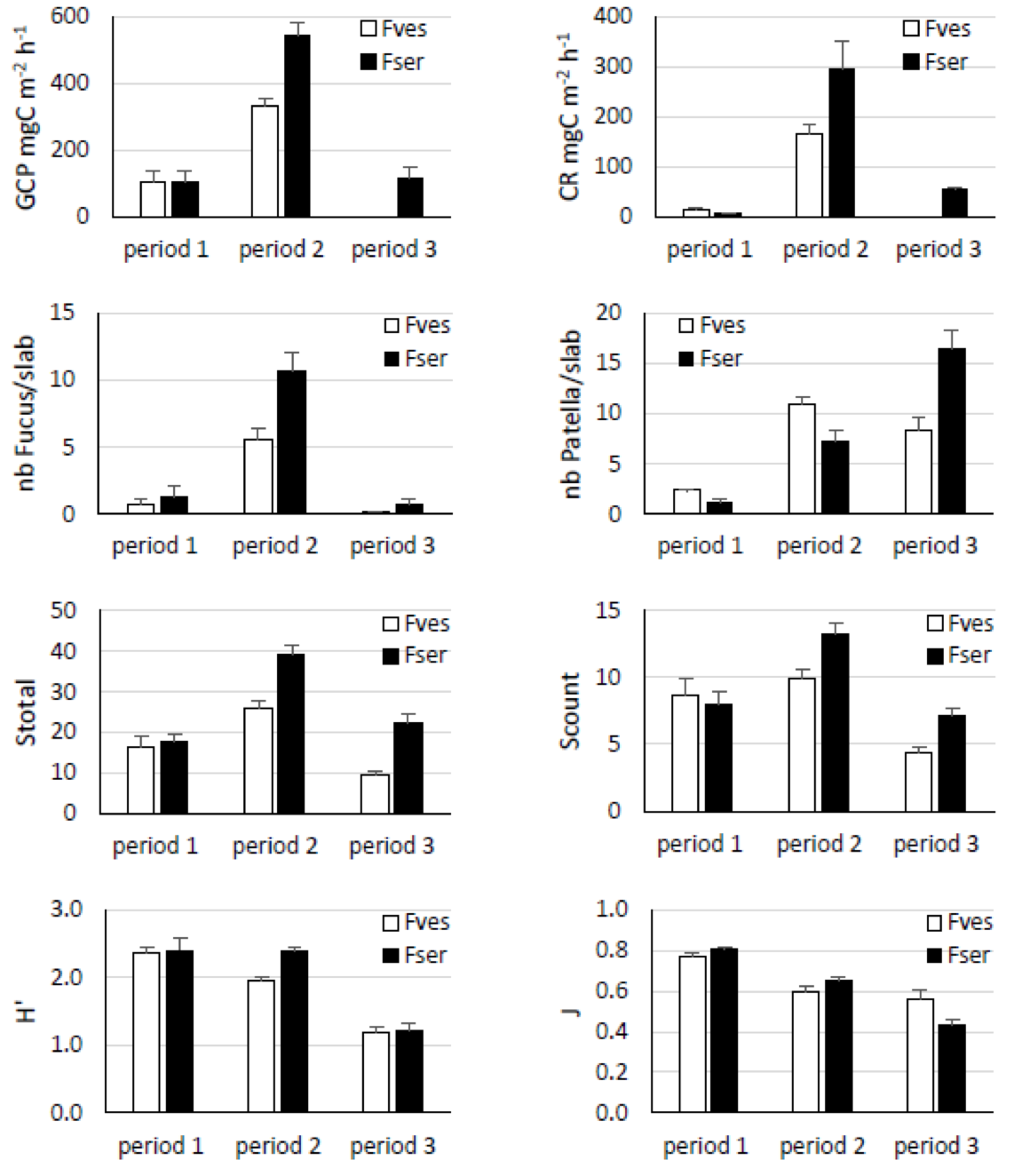
Figure 5 - Comparison of the means of the various parameters measured over the 3 periods of succession observed on slabs (0.4 x 0.4 m) set in the Fucus vesiculosus (Fves) and Fucus serratus (Fser) areas: gross community primary production (GCP), community respiration (CR), number of individuals of Fucus per slab, number of Patella per slab, total number of taxa on the 9 pooled slabs (Stotal), number of countable invertebrate taxa on the 9 pooled slabs (Scount), Shannon diversity index calculated based on countable invertebrate taxa on the 9 pooled slabs (H’) and Pielou’s evenness calculated based on countable taxa on the 9 pooled slabs (J). Error bars indicate standard errors (GCP and CR: n = 2, 17 and 0 for period 1, 2 and 3 in the F. vesiculosus area and 3, 12, 4 in the F. serratus area; nb Fucus, nb Patella, S, H’, J: n = 3, 15 and 7 for period 1, 2 and 3 in the F. vesiculosus area and 3, 11, 1 for period 1, 2 and 3 in the F. serratus area).
Insights from long-term survey
Placing bare substrata in two communities adjacent over the emersion gradient, we hypothesized that the timing of successional sequences would be slower in the mid intertidal than in the low-mid intertidal. Surprisingly, first Fucus individuals identifiable to the species level were observed 3 months sooner in the F. vesiculosus area (mid intertidal) than in the F. serratus area (low-mid intertidal). Moreover, those individuals belonged to the F. vesiculosus species in both areas and, at low-mid intertidal level, first F. serratus individuals were observed only 6 months after F. vesiculosus individuals. However, the two species fulfilled the same functional role in the community and one year after the slabs setting, as the surrounding communities, communities established on the slabs were characterized by high Fucus density, high taxa richness and high metabolic activity at both tidal levels. The transition between the first and the second period pointed above for this 10-year survey was concomitant in the two areas, that could invalidate our hypothesis. Nevertheless, the transition between the second and third period pointed above appeared later in the mid intertidal than in the low-mid intertidal (that is respectively 6- and 4-years post deployment of fouling plates). This confirmation of our hypothesis would not have been possible within the duration of most intertidal field experimental studies (Jenkins & Uya, 2016). The long-term observations made here following the provision of bare substrata revealed late transition from communities dominated by canopy-forming algae to grazing limpet dominated communities. Fucoid algae and grazing patellid limpet interactions are known to be greatly variable and to have various effects on the community structure of intertidal rocky shores of north west Europe (Jenkins et al., 2005). The present survey suggests that Fucus sp. and Patella sp. interactions changed over succession time, depending on the life-stage of the species and the development stage of the populations, and owing to the plasticity of the diet of the grazer (Schaal & Grall, 2015). Fucus sp. and Patella sp. interactions had direct and indirect effects on the structure and functioning of the whole macrobenthic communities, which also varied over succession time. During the first period, the few small individuals of Patella sp. that had recruited on slabs could either have had no effect on Fucus sp. recruitment or have facilitated it by grazing pioneer ephemeral algae. During the second period, Patella sp. and Fucus sp. individuals and populations grew on slabs concurrently until algae naturally decayed (the Fucus life-span being about 3 years). The limpet individuals and populations growth at that time could have been facilitated on slabs by the dampening effect of the Fucus canopies at emersion (amelioration of tide-out temperatures and relative humidity), particularly at the mid-intertidal level. In parallel, diverse flora and fauna also benefited from the canopies, due either to the shelter or to the substratum complexification they provided. Finally, community primary productivity was high during this second phase. The detachment of the Fucus sp. individuals from the slabs was thus accompanied by community taxa richness and primary productivity reductions, and also by a limpet mortality on slabs in the mid intertidal level. In the low-mid intertidal level, the emersion stress was weaker and limpets were still protected on slabs by the denser surrounding canopy. The numerous large individuals of Patella sp. on slabs were not suspected to outcompete Fucus sp. for space, but rather to prevent subsequent Fucus sp. recruitment by grazing germlings. Indeed, bare substrata persisted on slabs over the survey and the only Fucus sp. individuals remaining on slabs after 10 years had settled and developed on Patella sp. shells.
Communities established on slabs versus surrounding communities
Even made of natural materials and with enhanced roughness, artificial hard substrata are known to constitute poor surrogates of natural habitats that support assemblages different to those found on natural shores (Cacabelos et al., 2016). In particular, the experimental slabs used here offered less refuges for fucoid germlings to escape grazing than the surrounding natural boulder reef. At the end of the 10-year survey, communities established on slabs were dominated by limpets and differed from the surrounding communities dominated by fucoid algae at both tidal levels. The last year of the survey, the Patella sp. density was higher on slabs at mid-tidal level (about 40 ind m-2) than in the surrounding Fucus vesiculosus community (averaging 28 ind m-2 over the whole 10-year period, unpublished data) and much higher on slabs at mid-low tidal level (about 93 ind m-2) than in the F. serratus surrounding community (averaging 8 ind m-2, unpublished data). Limpets have long been shown to control Fucoid recruitment (Jones, 1946), and their important community regulating role as grazer has been widely documented on European rocky shores (Coleman et al., 2006). This community regulating role involves a complex series of interactions and depends on local environmental conditions. The threshold density beyond which limpets prevent the establishment of Fucus sp. might have been surpassed here on the experimental slabs (Jonsson et al., 2006). Further settlement of Fucus might thus depend upon a decrease in the limpet population as the adult limpets die off, and long-term cyclical changes of dominance between these two organisms, as documented in the Lough Hyne Marine Reserve of Ireland (Little et al., 2017), could occur. However, the peculiar environmental conditions experienced on artificial substratum potentially influence the structure of the colonising populations. For example, recent studies in the Irish Sea showed that dislodgement of Fucus vesiculosus was higher and that limpets were larger on artificial structures than on natural shores (Earp et al., 2023; Farrugia Drakard et al., 2021). Such effects could allow the limpet dominance to persist on the experimental slabs. Given the relatively long life-span of Fucus (about 3 years) and Patella (up to 15 years), very long-term (i.e. multi-decades) data series are required to test for cyclical changes of dominance. The persistence of limpet dominated communities or the alternance of limpet and fucoid dominated communities on the experimental slabs should then be checked by going on the survey for a further decade.
Acknowledgements
Thanks are due to Gabin Droual and Erwann Legrand for their help in setting up the heavy slabs on the shore and to Olivier Bohner for his assistance in data field acquisition. Preprint version 2 of this article has been peer-reviewed and recommended by Peer Community In Ecology (https://doi.org/10.24072/pci.ecology.100635; Bornette, 2024). The authors thank John Griffin for his constructive comments that contribute to the improvement of the paper.
Funding
This work benefited from the support of the Brittany Regional Council and the French National Research Agency through the Investments for the Future program IDEALG ANR-10-BTBR.
Conflict of interest disclosure
The authors declare that they comply with the PCI rule of having no financial conflicts of interest in relation to the content of the article.
Data, scripts and code accessibility
Data are available on line (https://doi.org/10.5281/zenodo.10401814; Migné, 2023).


 CC-BY 4.0
CC-BY 4.0