Introduction
Understanding and preventing biological invasions is crucial because they are among the leading causes of the ongoing biodiversity crisis, and threaten human health and societies (McGeoch et al., 2010; Pyšek & Richardson, 2010; Diagne et al., 2021). Human activities, by introducing species into new habitats, are the main cause of biological invasions globally (van Kleunen et al., 2015; Bonnamour et al., 2021). Among the many species transported by humans at global scale, some are able to establish and thrive in novel environments. They can potentially spread beyond their initial introduction point and cause damages, and are referred to as invasive alien species (Broennimann et al., 2007; Young et al., 2017; Pyšek et al., 2020; Fenn-Moltu et al., 2023). Insects for instance, because of their small size, are easily unintentionally transported by human activities through the contamination of traded materials or by hitchhiking on vehicles (Meurisse et al., 2019; Gippet et al., 2019). Species can also become invasive even within their native range by undergoing demographic explosions and rapidly expanding into new areas, thereby creating significant challenges (Valéry et al., 2009).
Invasive ant species are highly destructive, causing harm not only to biodiversity but also generating important socio-economic impacts (e.g. health issues, agricultural losses, costs of control and eradication) (Lach & Thomas, 2008; Silverman & Brightwell, 2008; Siddiqui et al., 2021; Tercel et al., 2023). For example, the fire ant Solenopsis invicta and the little fire ant Wasmannia auropunctata have cost billions of USD in damages and control efforts in the last decades (Angulo et al., 2022). Most invasive ant species can occupy various environments and exploit a large quantity and diversity of resources within their new environments (Crowder & Snyder, 2010; Felden et al., 2018; Balzani et al., 2021). Furthermore, these species often form polygynous and polydomous (i.e. multiple-queen, multiple-nest) colonies, conducive to the formation of supercolonies (Bertelsmeier et al., 2015; Helanterä, 2022). These supercolonies expand through budding, a process by which queens (and some workers) establish new nests close to the main colony while maintaining their connection to other nests by exchanging workers and thus preserving social recognition within the entire supercolony (Torres et al., 2007; Moffett, 2012; Helanterä, 2022). Supercolonies can cover large areas, with a high density of workers exploiting available resources (Gippet et al., 2018). For example, some supercolonies of Lasius neglectus can reach >20ha (Ugelvig et al., 2008; Gippet et al., 2022a).
The Tapinoma nigerrimum complex comprises five ant species, T. darioi, T. ibericum, T. magnum, T. hispanicum and T. nigerrimum, all of which originate from the Western Mediterranean area (i.e. from Spain to Italy and Morocco to Tunisia). These species are very similar morphologically and were considered to be a single species until the recent re-evaluation of this complex and its subsequent revision (Seifert et al., 2017, 2024). This complex comprises three polydomous species, which are capable of forming supercolonies (T. darioi, T. ibericum, T. magnum), and two monodomous species (T. nigerrimum and T. hispanicum) (Seifert et al., 2017, 2024). The three polydomous species have a great invasive potential as they can be common in anthropised environments and appear to be spreading through human activities (Seifert et al., 2017; Centanni et al., 2022; Lenoir et al., 2023). They also pose a threat to human activities and to native ants (Mansour et al., 2012; Gippet et al., 2022b; Gouraud & Kaufmann, 2022; Lenoir et al., 2023). Tapinoma nigerrimum and T. hispanicum which feature no traits associated with invasiveness, can respectively be found in southern France/northern Spain and southern/western Spain (Seifert et al., 2017, 2024). The probable native range of T. magnum encompasses eastern and southern Italy and North Africa (Seifert et al., 2017; Gouraud & Kaufmann, 2022). The situation in northern Italy and the Mediterranean coast of France is less clear, but may be due to an earlier range expansion of T. magnum (Seifert et al., 2024). Tapinoma darioi has a probable native distribution in North-East Spain and the French Mediterranean coast. Tapinoma ibericum is found in the southern and western Iberian peninsula including Spain and Portugal (Seifert et al., 2017; Gouraud & Kaufmann, 2022). In addition, these three species have been introduced into northern Europe. The first proven record for T. magnum out of its native range was in Edesheim, Germany in 2009 (Seifert et al. 2017) and the species is now present in Austria, Belgium, England, Germany, Switzerland and the Netherlands. Tapinoma darioi was detected in the Netherlands and T. ibericum in England, Germany and the Netherlands (Dekoninck et al., 2015; Seifert et al., 2017, 2024; Lenoir et al., 2023). These species are also invasive in several Mediterranean and northern French localities, where they exhibit invasive behaviour (Blight et al., 2010; Centanni et al., 2022; Gouraud & Kaufmann, 2022; Lenoir et al., 2023). The three invasive species are present in different proportions in the invaded areas, suggesting different patterns of introduction. In the urban area of Montpellier (Mediterranean France), for example, Centanni et al. (2022) show a strong abundance of T. darioi compared with the other two invasive species. On the other hand, in the northern parts of France and Europe, T. magnum is the most common species (Seifert et al., 2017; Lenoir et al., 2023).
The European spread of invasive Tapinoma species is suspected to occur mostly through the trade in ornamental plants (Seifert et al. 2024, Gouraud & Kaufmann, 2022). For example, Tapinoma ants have been detected in pots of olive trees in Pays de la Loire (north-west France) and Gironde (west France) departments (Gouraud & Kaufmann, 2022; Lenoir et al., 2023). Yet, the role of the trade in ornamental plants in shaping ongoing invasions of Tapinoma species remains unexplored. More generally, because it is still incipient, studying this invasion could provide invaluable insights into one of the most common pathways for insect invasions.
In this study, we focused on the ongoing invasion of T. darioi, T. ibericum and T. magnum in Western Europe. Our objective was to evaluate the role of Ornamental Plant Sales Outlets (OPSOs) as an introduction pathway for ant invasions using genetic tools, with a particular focus on the influx of species into OPSOs. We hypothesize that these species are more frequent in OPSOs than in surrounding areas. We also expect OPSOs to host non-native populations and act as hubs for invasion. To test these hypotheses, we collected and genotyped Tapinoma workers from OPSOs located in four urban areas of metropolitan France and one locality in Corsica with a specific focus on the Montpellier urban area (Mediterranean coast). We then analysed their relationships to workers from a large number of localities of the distribution range of the three species. This allowed us to determine species identity, define genetic groups within species and infer the broad geographical origins of the Tapinoma ants found in OPSOs.
Material and methods
Study sites and sampling
Systematic sampling of OPSOs was carried out in three French urban areas (Montpellier, Lyon, Mulhouse). Two opportunistic samples from OPSOs in Nantes urban areas and southern Corsica were added to the dataset (Figure 1A). The OPSOs were selected to represent a broad range of plant sellers (plant nurseries or garden centres). OPSO sampling was carried out by different teams of at least two people at different times of the day in the five urban zones resulting in different protocols described below.
In the Montpellier urban area (French Mediterranean bioregion) 24 OPSOs were sampled in March-April 2023. Two sampling perimeters were defined for each OPSO. The indoor perimeter consisted of all areas where the activities of the retailers were concentrated (greenhouses, outdoor pots, storage and composting areas). The outdoor perimeter surrounded the indoor perimeter and extended over 15 metres. Within each of the two sampling perimeters, 20 baits consisting of a strip of paper (11x4cm) on which a drop of honey were randomly distributed (Delabie et al., 2021). After one hour, the workers found on the baits were collected manually or using a mouth aspirator. Baiting was combined with one hour of visual prospection during which all the Tapinoma workers detected were collected. Several Tapinoma samples were analysed for each OPSO.
In the Lyon urban area (South of French continental bioregion) 61 OPSOs were sampled in spring 2019 and in the Mulhouse urban area (North of French continental bioregion) 16 OPSOs were sampled in summer 2019. The OPSOs were searched by visual prospection inside and outside, in all areas open to the public including greenhouses and open air plant nurseries. Only one Tapinoma sample was collected per OPSO in Lyon, regardless of the perimeter, but in Mulhouse, two samples were collected from the same OPSO: one indoors and one outdoors.

Figure 1 - (A) Location of the French urban areas where OPSOs were sampled, with disk size proportional to the number of OPSOs collected (ranging from 1 (Nantes and Southern Corsica) to 61 depending on the urban area). (B) Distribution of samples of T. darioi (orange dots), T. ibericum (purple triangles), T. magnum (green squares) collected in different localities of western Europe and North Africa and used in this study.
In the Nantes urban area (Oceanic bioregion) and southern Corsica (Mediterranean Island), only one sample was collected by visual prospection in 1 OPSO in each urban area (Figure 1A).
In parallel, from 2009 to 2023, Tapinoma workers were collected during systematic (e.g. Centanni et al. (2022)) and opportunistic sampling at various sites in Europe and North Africa, which we will refer to as “localities” in the remainder of this manuscript (Figure 1B).
Data from Seifert et al. (2024), from the present article, and from records from Greece (which were identified to species by genotyping but not used in the OPSO analysis) were used to draw maps of the invasive status of T. darioi, T. ibericum and T. magnum in Europe and the Mediterranean (Figure 2, Appendix 1). Six categories were considered, based on the following criteria:
NO DATA: no record in Seifert et al. (2024) or in the present study
NATIVE: distribution continuous, strong majority of records of the species compared to other supercolonial Tapinoma species and Mediterranean plant export (i.e. export of Mediterranean plants such as potted mature olive trees, which have been cited as invasion vectors for the species group (Seifert et al., 2017))
PROBABLY NATIVE: at the edge of the continuous NATIVE distribution, Mediterranean plant export but few records, some records of other supercolonial Tapinoma species
UNCERTAIN: at the edge of the continuous NATIVE distribution, Mediterranean plant export but records of other supercolonial Tapinoma species are at least as frequent
PROBABLY EXOTIC: at the edge of continuous NATIVE distribution or discontinuous, with a strong majority of records of other supercolonial Tapinoma
EXOTIC: distribution is discontinuous from the NATIVE distribution or not Mediterranean.
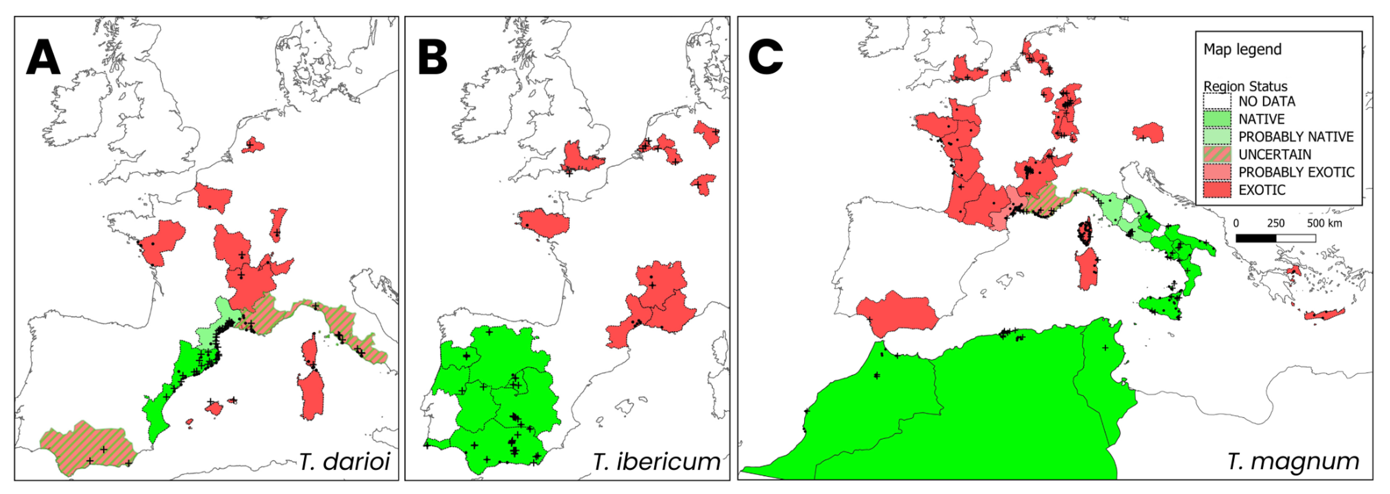
Figure 2 - Maps of the known distribution and native/exotic status of (A) T.darioi, (B) T. ibericum and (C) T. magnum. Crosses represent records from Seifert et al. (2024), dots represent records which were identified by microsatellite genotyping in the present study and in Greece. Crosses and dots overlap when records were both genotyped and used in Seifert et al. (2024). Colors indicate native/exotic status of European NUTs –level 2 regions, English regions and North-African countries. Bright green indicates NATIVE status, muted green PROBABLY NATIVE, hatched green/red UNCERTAIN status, muted red PROBABLY EXOTIC, bright red EXOTIC.
We used the NUTS 2024 layer for regional boundaries of continental Europe (NUTS level 2, https://ec.europa.eu/eurostat/web/gisco/geodata/statistical-units/territorial-units-statistics), the december 2023 regions layer for England (https://geoportal.statistics.gov.uk/datasets /regions-december-2023-boundaries-en-bfc-2/explore) and the World Administrative Boundaries dataset for Algeria, Morocco and Tunisia (https://public.opendatasoft.com/explore/dataset/world-administrative-boundaries/export/).
All statuses are hypotheses based on our current knowledge of the distribution of species. They might change with additional records and an in-depth population genetic approach, which is outside the scope of the present article.
DNA extraction and Genotyping
DNA was extracted from workers of the Tapinoma nigerrimum complex collected from OPSOs (n = 234) and from different localities (n = 486) by grinding each worker in proteinase K (10 µL at 15 mg/ml) and Chelex 7% (150 µL). After grinding, samples were incubated at 55°C for 2 hours, followed by a further 15 minutes at 90°C to inactivate the proteinase K.
To identify the species of individuals collected and to assign them to genetic groups, 15 microsatellite markers specifically developed for the Tapinoma nigerrimum complex were selected and pooled into three genotyping multiplexes (Centanni et al., 2022). The three PCR mixes consisted of 6 μl of Master Mix Type-it microsatellite PCR kit (QIAGEN, 206246, Hilden, France), 3.6 μl of H2O, 2 μl of DNA from each sample collected and 0.4 μl of forward primer + reverse primer + tails. Forward primers were end-labelled with universal tails according to Blacket et al. (2012), using fluorescent dyes. PCR of the three genotyping mixes was carried out using the following PCR programme: 5 min denaturation at 95°C, then 32 cycles with denaturation (30 s) at 95°C, annealing (3 min) at 60°C, and extension (30 s) at 72°C, and a final extension of 30 min at 60°C. Finally, a 30-minute migration of the PCR products on an agarose gel (3%) was carried out to check the quality of the amplification. All the PCR products were then analysed in a 3730xl DNA analyser (Applied Biosystems) by a service provider (GENTYANE, Clermont-Ferrand, France). Electropherograms were read and interpreted using Genemarker 1.95 software (Softgenetics, State College, PA, USA).
Species identification and comparison of occurrences
Genetic clusters using all individuals were defined using a combination of two analytical methods: simple PCA (Jombart et al., 2010) and Bayesian clustering (STRUCTURE program, Pritchard et al., 2000), using a similar method as Centanni et al. (2022). Species names were assigned to these clusters based on the multilocus microsatellite genotypes of reference individuals identified in Seifert et al. (2017).
In the present study, an occurrence was defined as the presence of at least one individual of a species in an OPSO or in one point of the standardised grid in Centanni et al. (2022). We tested whether the proportion of occurrences of species of the Tapinoma nigerrimum complex differed between outdoor and indoor perimeter of the OPSOs using a χ² compliance test. We also tested if OPSOs samples were similar to samples from Centanni et al. (2022) with an additional χ².
Assessment of OPSOs population structure and identifying their source populations
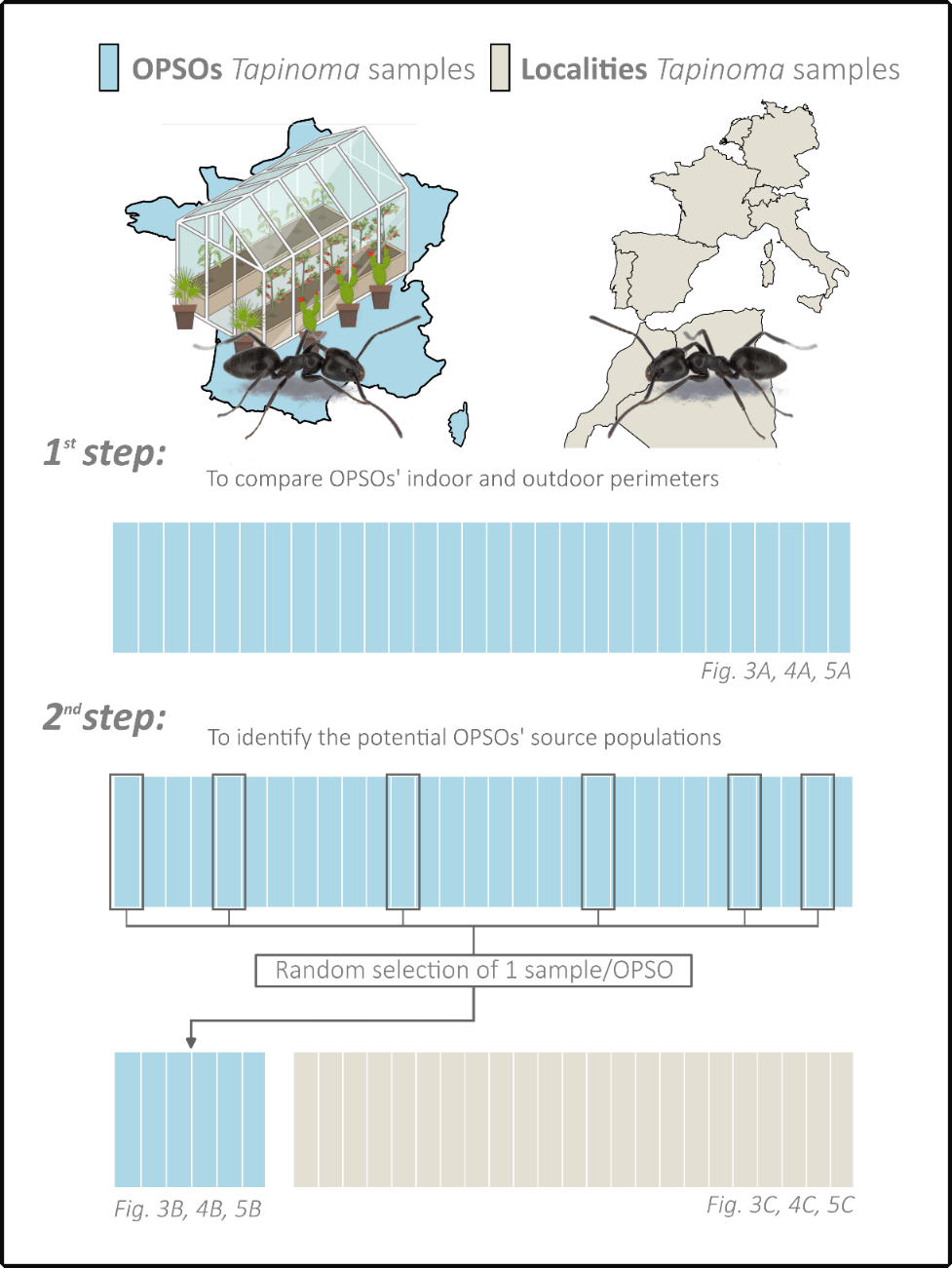
The genetic structure of the populations within each species was analysed by Bayesian clustering using STRUCTURE 2.3.4 (Pritchard et al., 2000). Clustering analysis was realised in two steps, consisting in a series of three analyses, one for each Tapinoma species (Appendix 2). Firstly, a STRUCTURE analysis was performed using only the OPSOs samples to determine whether there were one or more genetic groups within each OPSO and to know if the outdoor populations were the same as the indoor populations. Then, a second STRUCTURE analysis was performed in order to identify the potential source populations of the OPSOs. For this second step, we used a dataset consisting of samples from various localities in Western Europe and North Africa. This dataset included either 179 samples of T. darioi species, 234 samples of T. magnum species, or 30 samples of T. ibericum species, which served as the reference source populations. We included one individual (selected randomly) per genetic group and per OPSO, which were the specimens whose origin was to be determined. Not all specimens from OPSOs were used to avoid potential bias in Bayesian analysis. For each STRUCTURE analysis, an admixture model was built using a burn-in period of 250,000 iterations, followed by 250,000 MCMC (Markov Chain Monte Carlo) iterations. For each potential number of genetic groups, denoted as K (ranging from 1 to 10) (Pritchard et al., 2000), a total of 15 independent runs were executed. For each STRUCTURE analyses the K values were selected using three criteria : high Evanno’s ΔK (Earl & von Holdt, 2012), high mean similarity between the runs and small number of minor clusters. The admixture models obtained from these analyses were then compiled using CLUMPAK (Kopelman et al., 2015) which provides consensus between similar runs generated by STRUCTURE. The spatial distribution of genotypes for samples from the various localities was then mapped out using QGIS 3.28.3, using the compilation of the runs from STRUCTURE built in CLUMPAK during the second analysis.
Results
Occurrences of Tapinoma species in OPSOs
An important proportion of OPSOs featured Tapinoma species (37.8%). Where systematic sampling was carried out, the presence of Tapinoma species in OPSOs varied strongly depending on urban areas: 70.8% in Montpellier; 31.1% in Lyon; 6.3% in Mulhouse. Over the 103 sampled OPSOs, T. magnum was the most frequently found with 30 occurrences (29.1%), T. darioi was found in 11 OPSOs (10.7%), T. ibericum in 7 OPSOs (6.8%) and T. nigerrimum 3 OPSOs (2.9%) (Figure 3A).
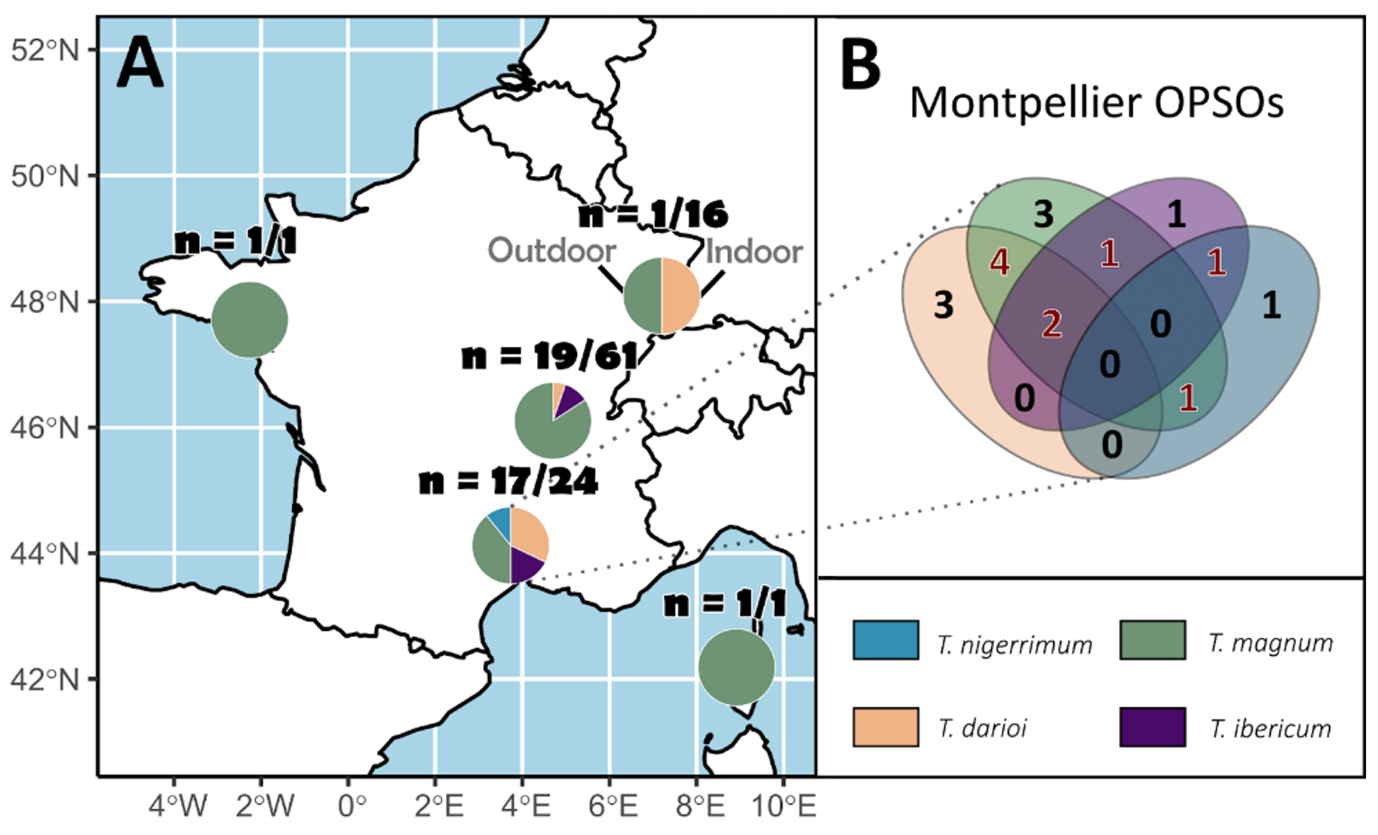
Figure 3 - (A) Proportion of occurrences of Tapinoma nigerrimum complex species in French OPSOs (n = number of OPSOs colonised / total number of OPSOs visited) (B) Venn diagram showing occurrences (black numbers) and co-occurrences (red numbers) of T. magnum, T. darioi and T. ibericum in 17 Montpelier OPSOs (in the other areas only one sample was collected per OPSO, and thus, co-occurrences could not be investigated).
Occupancy of baits by Tapinoma species in Montpellier
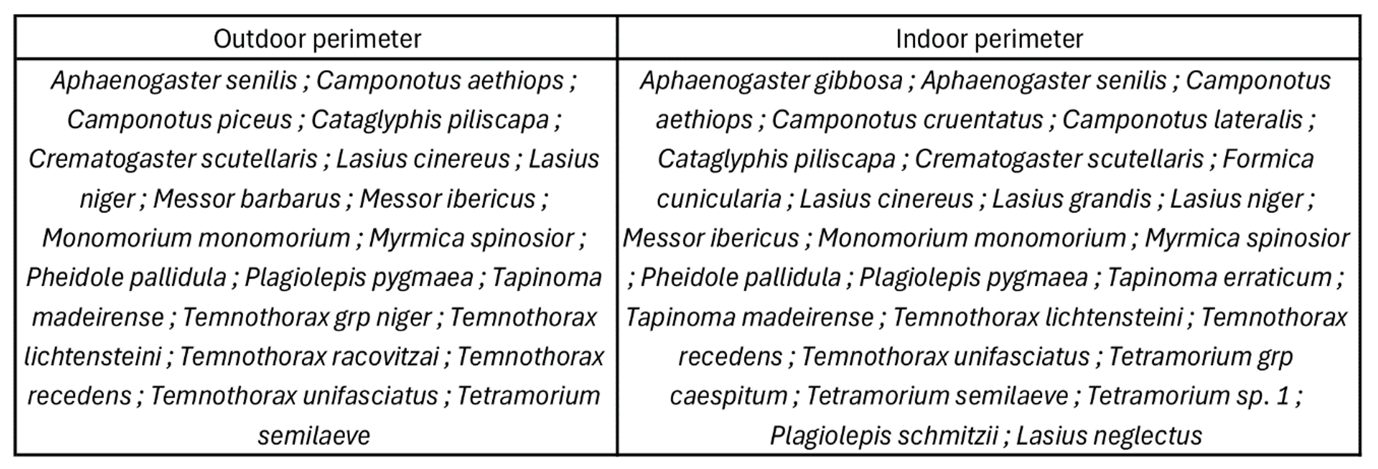
Among the baits of 24 OPSOs in Montpellier, an average of 5.5 baits out of 20 were visited by ants indoors, and 6.5/20 outdoors. Among these baits, a total of 30 species were collected, with 26 species indoors and 20 species outdoors (Appendix 3). Among these 30 species, two were unambiguously exotic: Plagiolepis schmitzii and Lasius neglectus. These two species were present in only 1% of the occupied baits. Species of the Tapinoma nigerrimum complex were found on 14.8% of the occupied baits, while other species occupied 85.2% of the baits. The majority of Tapinoma nigerrimum complex workers collected for the study were sampled through active prospection (78%).
Occurrences of Tapinoma species in Montpellier and Lyon
Seven OPSOs were colonised simultaneously by more than one invasive Tapinoma species: 2 by T. darioi, T. ibericum and T. magnum, 4 by T. darioi and T. magnum and 1 by T. ibericum and T. magnum. Tapinoma nigerrimum was also found co-occurring, once with T. ibericum and once with T. darioi. (Figure 3B). The indoor perimeters of 17/24 OPSOs were colonised by at least one species from the Tapinoma nigerrimum complex. In the 24 OPSOs, there were 8 occurrences (one occurrence = at least one individual of one species regardless of the sampling method in one OPSO) of T. darioi (32%), 5 occurrences of T. ibericum (20%), 10 occurrences of T. magnum (40%), and 2 occurrences of T. nigerrimum (8%). The outdoor perimeter of 14/24 OPSOs presented at least one Tapinoma nigerrimum complex species: 8 occurrences of T. darioi (47%), 1 occurrence of T. ibericum (5.9%), 6 occurrences of T. magnum (35.3%), and 2 occurrences of T. nigerrimum (11.8%). The relative proportions of the four species discovered in the indoor and outdoor perimeter OPSOs did not differ significantly (χ = 0.41, P = 0.82). In the Montpellier OPSOs a total of 211 workers of the Tapinoma nigerrimum complex were genotyped, consisting of 111 T. darioi, 19 T. ibericum, 70 T. magnum used for STRUCTURE analysis (1 individual corresponding to 1 bar) and 11 T. nigerrimum (Appendix 1).
In contrast, in the localities of Montpellier (not taking into account OPSOs sampling), for 926 sampling point, occurrences were as follows: 78 T. darioi (27.8%), 1 T. ibericum (0.3%), 6 T. magnum (2.1%) and 197 T. nigerrimum (69.8%) (Centanni et al., 2022). The relative proportions of the four species discovered in the indoor and outdoor perimeter OPSOs differed significantly from those sampled in Montpellier localities (χ = 20.32, P = 0.00004 for indoors and χ = 6.90, P = 0.03 for outdoors). The proportions of T. magnum, T. darioi and T. ibericum were higher than expected, while the number of T. nigerrimum was lower than exempted in OPSOs.
In the 31.1% OPSOs colonised in Lyon, there were 1 occurrence of T. darioi, 2 occurrences of T. ibericum and 16 occurrences of T. magnum. In the localities of Lyon (not taking into account OPSOs sampling), for 1248 sampling points, occurrences were as follows: 0 T. darioi, 0 T. ibericum, 2 T. magnum (unpublished data from Gippet et al., 2017).
Structure of Tapinoma darioi populations and putative source populations of OPSOs
Focusing the analysis on the 9 OPSOs sampled in Montpellier, the most likely number of genetic clusters (K) detected for T. darioi was 4. However, this clustering exhibited multimodality (i.e. different runs of STRUCTURE resulting in alternative distribution of individuals among clusters) with two modes (major mode with 10/15 runs). It remained the most likely (highest Evanno score) and other values of K also had multiple modes. Only a single cluster was detected in each OPSO, except for OPSO 21 where cluster 3 was detected outdoor and cluster 3 and 4 indoor (Figure 4A). The broader analysis using T. darioi samples from all localities (Figure 4C) plus one individual per genetic cluster per Montpellier OPSO as defined above, and individuals of other OPSOs (Figure 4B) suggested a most likely K = 6, despite exhibiting two modes (major mode with 14/15 runs).
The most common cluster in localities (K1, light purple, Figure 4C), especially along the Spanish coast and outside the Mediterranean area, was detected in two OPSOs in Montpellier and one in Lyon. The second most common cluster (K3, orange, Figure 4C) was found in Montpellier localities only (with a clustered distribution in the South-West of the Montpellier area), and was detected in one Montpellier and one Mulhouse OPSO. The third most common cluster (K6, blue) distributed all along the French Mediterranean coast localities was detected in one Montpellier OPSO. The fourth most common cluster (K5, dark purple, Figure 4C) was common in Montpellier localities, and was detected in 3 Montpellier OPSOs. The fifth most common cluster (K2, beige) present in Italy, Corsica and with a few occurrences in Montpellier was not found in the sampled OPSOs. The less common cluster (K4, green) was found in Montpellier localities only, and was detected in 3 Montpellier OPSOs (Figure 4B, 4C).
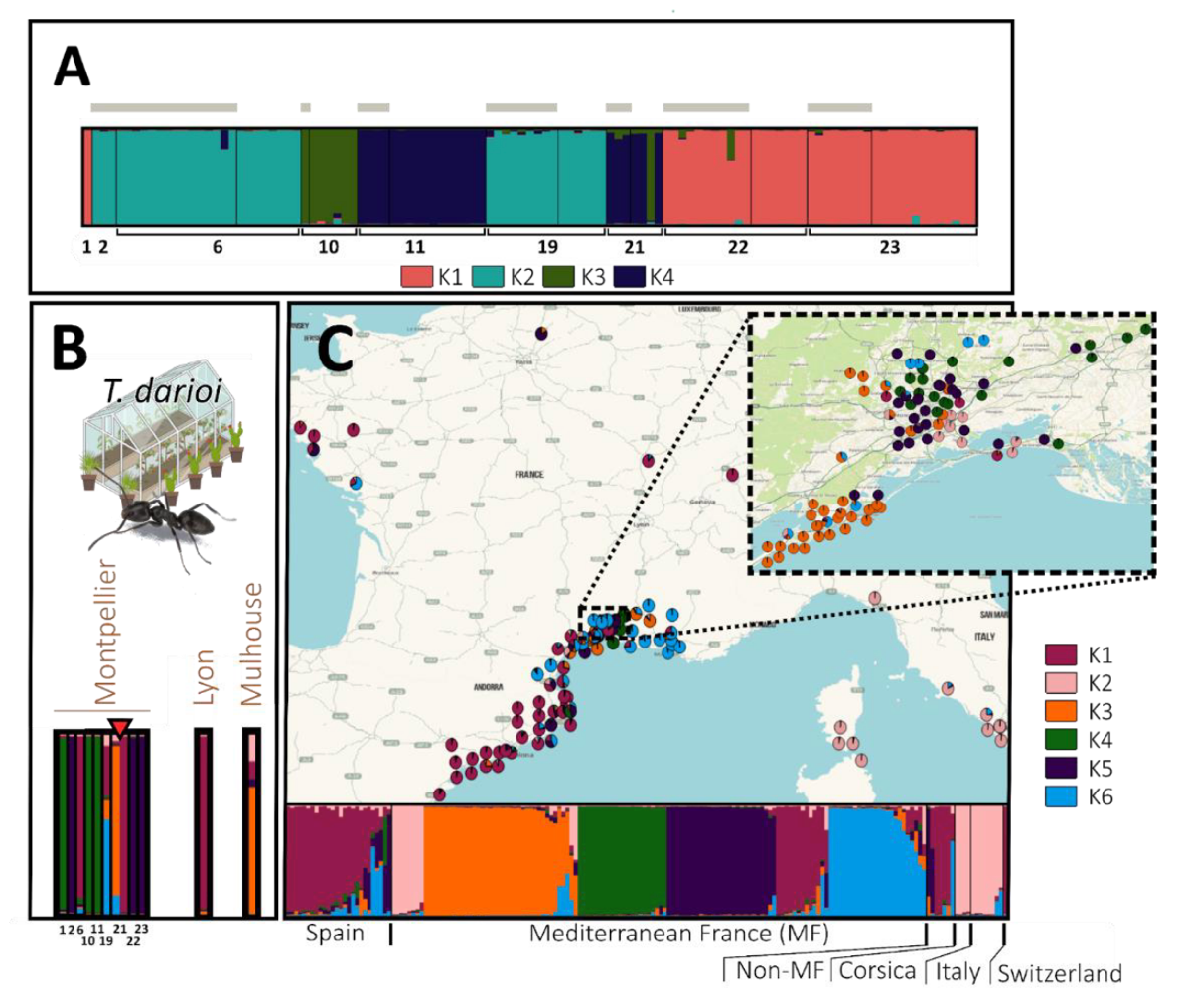
Figure 4 - Population genetic structure of T. darioi. In barplots, each individual is represented by a vertical line, which is divided into coloured segments representing the membership coefficient to each genetic group, e.g. Mulhouse (B) has 0.7 membership in cluster K3, 0.15 in K2, 0.1 in K1 and 0.05 in K5 (one colour per group). (A) STRUCTURE analysis including all individuals sampled in the OPSOs of Montpellier (K = 4). Numbers below the barplot correspond to the codes of the sampled OPSO. Individuals sampled outdoors of OPSOs are indicated with a grey horizontal bar, while individuals sampled indoors are not. (B) STRUCTURE analysis including one individual by OPSO and by genetic group (with a red triangle indicating OPSO colonised by samples of several genetic groups) and (C) all individuals sampled in the various localities (K = 6). The STRUCTURE bar plots of the individuals from the OPSOs (B) and the localities (C) were produced in the same structure analysis. Individuals collected in OPSOs are not included in the map.
Structure of Tapinoma ibericum populations and putative source populations of OPSOs
Focusing the analysis on the Montpellier OPSOs, the most likely number of genetic clusters detected for T. ibericum in the 5 OPSOs was 2; for this value of K there was only one mode. Only one single cluster was detected in each OPSO (Figure 5A). A broader analysis using samples from all localities (Figure 5C) plus one individual per genetic cluster per Montpellier OPSO, and individuals of other OPSOs (Figure 5B) suggested a most likely K = 3, despite exhibiting three modes (major mode with 9/15 runs).
The most common cluster in localities (K1, blue, Figure 5C), especially in northern Spain, central Spain and Portugal, was detected in 3 Montpellier OPSOs. The second most common cluster (K3, orange) was found in southern Spain, and was detected in 2 Montpellier and 2 Lyon OPSOs (Figure 5B, 5C).
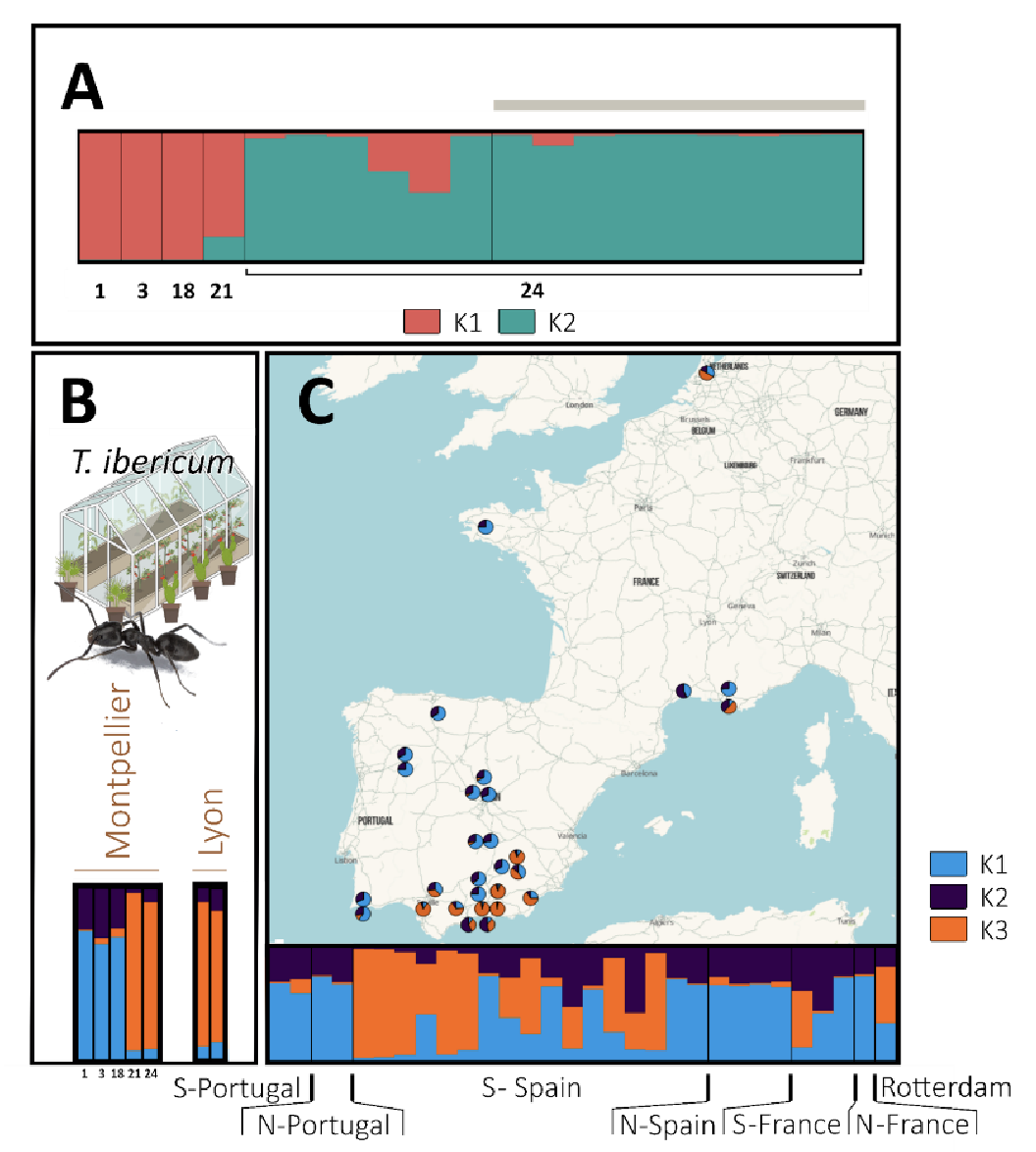
Figure 5 - Population genetic structure of T. ibericum. In barplots, each individual is represented by a vertical line, which is divided into coloured segments representing the membership coefficient to each genetic group (one colour per group). (A) STRUCTURE analysis including all individuals sampled in the OPSOs of Montpellier (K = 2). Numbers below the barplot correspond to the codes of the sampled OPSO. Individuals sampled outdoors the OPSOs are indicated with a grey horizontal bar, while individuals sampled indoors are not. (B) STRUCTURE analysis including one individual by OPSO and by genetic group and (C) all individuals sampled in the various localities, with S = South and N = North (K = 3). The STRUCTURE bar plots of the individuals from the OPSOs (B) and the localities (C) were produced in the same structure analysis. Individuals collected in OPSOs are not included in the map.
Structure of Tapinoma magnum populations and putative source populations of OPSOs
Focusing the analysis on the Montpellier OPSOs, the most likely number of genetic clusters detected for T. magnum in the 11 OPSOs was 7, for this K there was only one mode. In 5 out of 11 Montpellier OPSOs (OPSOs number 2, 5, 10, 19, 20), more than a single genetic group was identified (between 2 and 5 genetic groups). For the Montpellier OPSOs, the number of genetic groups detected were generally higher indoor than outdoor. The genetic groups found in the outdoor perimeter were detected in the indoor perimeter as well, except for OPSO number 10, where samples found outdoor and indoor belonged to different genetic groups (Figure 6A).
A broader analysis using samples from all localities (Figure 6C) plus one individual per genetic cluster per Montpellier OPSO as defined above, and individuals of other OPSOs (Figure 6B) suggested a most likely K = 7, despite exhibiting two modes (major mode with 9/15 runs).
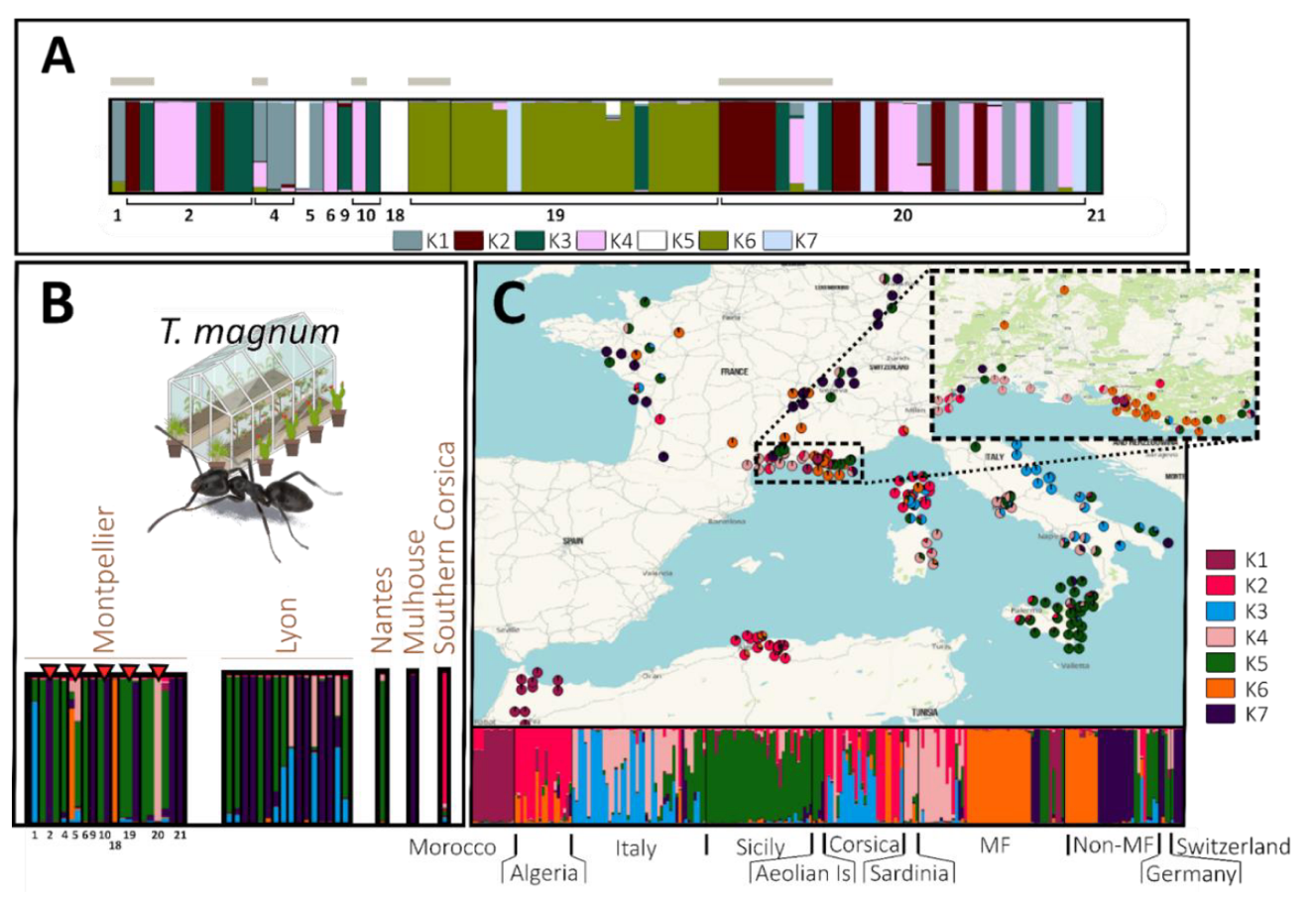
Figure 6 - Population genetic structure of T. magnum. In barplots, each individual is represented by a vertical line, which is divided into coloured segments representing membership coefficient to each genetic group (one colour per group). (A) STRUCTURE analysis including all individuals sampled in the OPSOs of Montpellier (K = 7). Numbers below the barplot correspond to the codes of the sampled OPSO. Individuals sampled outdoors the OPSOs are indicated with a grey horizontal bar, while individuals sampled indoors are not. (B) STRUCTURE analysis including one individual by OPSO and by genetic group (with a red triangle indicating OPSO colonised by samples of several genetic groups) and (C) all individuals sampled in the various localities (K = 7). The STRUCTURE bar plots of the individuals from the OPSOs (B) and the localities (C) were produced in the same structure analysis and have the same cluster colour code. Individuals collected in OPSOs are not included in the map.
Overall, clustering OPSOs with localities suggested diverse geographical origins of ants found in OPSOs. All clusters found in putative native zones (i.e. Italy, North Africa, clusters K1, K2, K3, K4, K5 and K7) were detected in OPSOs, except for K1. Clusters K5 and K7, found in Sicily and south-eastern Italy respectively, were highly prevalent in both OPSOs and non-Mediterranean France localities. Cluster K6 was common in Mediterranean and non-Mediterranean France, especially in Lyon, but only found in 2 OPSOs in Montpellier. Most Algerian individuals had partial membership in this cluster, suggesting a close relationship (Figure 6B, 6C).
Discussion
In this study, we investigated the occurrence of three invasive species of the Tapinoma nigerrimum complex within OPSOs in Mediterranean and non-Mediterranean French areas, as well as the role of OPSOs in their introduction into new localities. Microsatellite markers were used to identify Tapinoma species within OPSOs and to analyse their genetic structure. This analysis involved worker samples from OPSOs in four urban areas of metropolitan France and one locality in Corsica, as well as from various locations in Europe and North Africa. Invasive Tapinoma, most frequently T. magnum, were found in 37 out of 101 OPSOs inspected, supporting the hypothesis that the ornamental plant trade could play a role as a hub for the introduction of Tapinoma ants in France. Additionally, some of these OPSOs were colonised by several Tapinoma species simultaneously. The potential source populations of the invasive Tapinoma species found in the sampled OPSOs seemed to be diverse for T. magnum and T. darioi, indicating multiple geographic origins. Additionally, some OPSOs housed multiple populations of the same species.
Spread dynamic of Tapinoma induced by OPSOs
First of all, we observed that the percentage of colonised OPSOs and the presence of invasive Tapinoma species differed across cities, with a high percentage in Montpellier, an intermediate level in Lyon, and a low percentage in Mulhouse. This difference could be explained by the introduction of material from the native regions of these species. In particular, Montpellier probably imports more Mediterranean plants than Mulhouse due to geographic proximity and climatic similarity, and thus, has a higher probability of introducing Tapinoma. Additionally, we can assume that the climate in Mulhouse is less favorable for Tapinoma, which are Mediterranean species (Seifert et al. 2024), making their maintenance in and around OPSOs more challenging. Nonetheless, the sampling of Mulhouse OPSO was conducted during the summer, a period when Tapinoma is less active than in spring (Destour et al., 2024a). Consequently, we likely underestimated the presence of Tapinoma in this locality.
We observed that T. magnum, T. darioi and T. ibericum were more frequent in the OPSOs of Montpellier relative to the respective species regional distribution revealed by the systematic sampling campaign of Centanni et al. (2022) (which did not encompass OPSOs, but urban environments such as parks or streets). Similarly, T. magnum was common in Lyon OPSOs (16/61), but only 2 samples out of 1,248 sampling points were found during a systematic sampling in the area (Gippet et al., 2017). This indicates a strong propagule pressure of T. magnum caused by OPSOs. Tapinoma darioi and T. ibericum were less common in the Lyon OPSOs (1 and 2 occurrences respectively out of 61 OPSOs), but were not found during systematic sampling in the locality (Gippet et al., 2017). Therefore, OPSOs were potentially involved in the spread of T. magnum, T. darioi and T. ibericum in several localities in France outside the Mediterranean zone. Nonetheless, we should consider that there is a significant time gap between the sampling of the Montpellier and Lyon OPSOs and the surrounding localities, with the OPSOs sampled later in both cases. The expansion of invasive ants can occur rapidly, as demonstrated by Lasius emarginatus in New York (Kennett et al., 2024), where noticeable spread took place within just a few years. Therefore, the observed difference in frequency between invasive Tapinoma species in OPSOs and surrounding localities may be partly explained by this temporal gap, where an overall increase in frequency in both areas over time could lead to an apparent overrepresentation in OPSOs.
In Montpellier we observed that when a genetic cluster was present in the indoor perimeter of an OPSO for the three invasive species, it was also found in the outdoor perimeter. This suggests that Tapinoma species might be able to leave the buffered indoor conditions of OPSOs to forage in the natural surrounding environment and potentially establish themselves after being introduced through the plant trade. Alternatively, they could also colonize indoor environments from outdoor ones. Indeed, invasive species of the Tapinoma nigerrimum complex seem to be particularly flexible in terms of environmental conditions, as shown in T. magnum which is able to rapidly acclimate to new thermal conditions in a context of invasion (Bujan et al., 2021).
Tapinoma magnum seems however to struggle to colonise the Montpellier region, despite its ability to acclimatise to novel climatic conditions (Bujan et al., 2021). This observation could result from niche and habitat saturation by sister species, in particular T. darioi and T. nigerrimum. Destour et al. (2024a) showed that T. darioi and T. magnum forage at similar temperatures. In Montpellier, given the high abundance of T. darioi (Centanni et al., 2022), the available niches could potentially be saturated by this species, or other species native to the Mediterranean region, which would be a competitor for T. magnum. This would limit the spread of T. magnum in Montpellier, in the same way as species of the Tapinoma nigerrimum complex could limit the spread of L. humile in Corsica by competitive exclusion (Blight et al., 2010). In the case of the Tapinoma species, this remains quite speculative at this stage. The differences could also stem from varying introduction and invasion dynamics between the two species.
In Montpellier OPSOs, we observed a relatively low number of occurrences of T. nigerrimum, the species of the complex that is non-invasive and native to this area. Tapinoma nigerrimum represented only 10.7% of the occurrences of species of the Tapinoma nigerrimum complex in the OPSOs, whereas in the Montpellier localities T. nigerrimum represented 70% of the occurrences of species of the complex (Centanni et al., 2022). The low occurrence of T. nigerrimum in the OPSOs may be due to its inability to form supercolonies, reducing its chances of infecting soils and plants with a mated queen capable of founding a new colony. In addition, in the OPSOs of its native range, T. nigerrimum may be excluded by the other three species of the complex after their introduction (Holway et al., 2002). In fact, a higher proportion of the three invasive species of the Tapinoma nigerrimum complex (T. darioi, T. ibericum, T. magnum) was observed in the Montpellier OPSOs compared to the Montpellier localities. T. nigerrimum is also less abundant in urbanised environments, where OPSOs are located (Centanni et al. 2022). Given all these observations, we assume that the presence of T. nigerrimum in OPSOs is most likely due to colonisation from the outdoors, while the presence of T. magnum and T. ibericum is attributed to plant transport, and the presence of T. darioi is probably due to both colonisation from the outdoors and plant transport.
We were able to identify potential source populations for the ants found in French OPSOs. The main localities hosting the potential source populations of the invasive Tapinoma sampled in the OPSOs are southern Italy for T. magnum and Spain for T. darioi and T. ibericum. This aligns with the origins of most plants in the Montpellier OPSOs (OPSOs staff, pers. comm.). These findings support and further detail the hypothesis that the invasive Tapinoma species found in western France originated from Spain and Italy, likely introduced through plant pots from these regions (Gouraud & Kaufmann, 2022; Lenoir et al., 2023). However, among the OPSOs sampled, we also identified populations of T. darioi and T. magnum, which are probably originating from the south of France, since their genetic clusters have been found only in southern France. Nevertheless, inferring putative source populations does not account for non-sampled populations. In our large-scale study, some populations may have been missed due to their wide geographical distribution area. This can be problematic if the non-sampled population acts as an invasion bridgehead (Estoup & Guillemaud, 2010).
Multiple introductions at the population and species levels
Despite having wings, the natural dispersal of supercolonial Tapinoma mated gynes usually takes place close to the home colony, where they seek adoption in conspecific nests (Seifert et al. 2024). Species using this type of dispersal, as opposed to those using mainly flight dispersal, disperse over shorter distances (Cronin et al., 2013), which is incompatible with the current distribution of the three species in Europe. Indeed, the dispersal mode of these species should result in a high level of spatial genetic structuration (Moffett, 2012). However, we observed that in T. magnum and T. darioi, several genetic groups could be found within a single OPSO, indicating a high frequency of long-distance transportation typical of human activities, such as the transportation of ornamental plants. Hence, OPSOs could be subjected to multiple introductions of different populations countering genetic founder effects (Dlugosch & Parker, 2008; Zalewski et al., 2010). Multiple introductions could therefore facilitate the establishment of invasive Tapinoma populations at invaded sites by increasing genetic diversity, as well as propagule pressure and spatial range (Ahlroth et al., 2003; Roman & Darling, 2007). A striking example of this process is the case of Solenopsis invicta in the USA, whose initial founding group colonising the country is estimated to have consisted of only 9 to 20 unrelated mated queens introduced into a city in Alabama (Ross & Shoemaker, 2008).
While detecting multiple introductions of Tapinoma populations, we observed the presence of several invasive species from the Tapinoma nigerrimum complex within individual OPSOs. The different species in this complex can therefore get in close contact through the transport of ornamental plants, which could lead these species to hybridise in the future, as suggested by Seifert et al. (2017). Hybridisation is a phenomenon that can increase the distribution areas of invasive species, in particular by increasing ecological niche breadth (Pfennig et al., 2016; Carscadden et al., 2020). This phenomenon could weaken the Allee effect responsible for failure of a large number of biological invasions due to reduced individual fitness in small populations (Yamaguchi, 2004; Mesgaran et al., 2016). Hybridization can also help to increase fitness and invasive potential by improving biological traits such as fertility (Schierenbeck & Ellstrand, 2009; Hovick & Whitney, 2014). For example, in the invasive ant species Solenopsis geminata and Solenopsis xyloni, hybrids show a competitive advantage over pure species, with hybrids being more aggressive than S. xyloni and foraging more efficiently than S. geminata (Axen et al., 2014). In species within the Tapinoma nigerrimum complex, hybrids, as in Solenopsis, can produce fertile offspring (Fournier & Aron, 2021). Therefore, we might also expect to find competitive advantages in hybrids that would enhance their invasive characteristics (Seifert et al., 2017; Fournier & Aron, 2021).
Management implications
Controlling the invasion routes and associated vectors remains the most appropriate strategy for limiting biological invasions and mitigating their effects (Hulme, 2009; Pergl et al., 2017). OPSOs make up the most visible part of the global plant trade, but large landscaping operations such as new urban green spaces import plants through bulk importers or directly from abroad. In addition, invasive ants can be introduced through various other means such as soil transport, food product shipment, or even vehicular transportation (Suhr et al., 2019; Siddiqui et al., 2021; Hsu et al., 2024). The establishment of these species in countries like the Netherlands, a global leader in the production and export of ornamental plants, could result in worrying large scale secondary spread and bridgehead effect (Van Tuyl, 2012; Javal et al., 2019; Blumenfeld et al., 2021). Additionally, these species are thriving in anthropised habitats such as urban areas (Centanni et al., 2022), which could potentially facilitate their invasion of other similarly human-modified environments as suggested by Hufbauer et al. (2012) («Anthropogenically Induced Adaptation to Invade», AIAI) and shown in the little fire ant, Wasmannia auropunctata (Foucaud et al., 2013).
Monitoring OPSOs for invasive ant species could be part of routine controls which are mandatory in Europe for some plant diseases and pests (e.g. The Asian long-horned beetle Anoplophora glabripennis) (Commission Delegated Regulation (EU), 2019). However, OPSOs staff lack the training, financial resources, and technical capabilities to carry out invasive species checks within their live plants. As a consequence, OPSOs and other centres of exotic plant importation may serve as a pathway for many other invasive species besides Tapinoma (Parrella et al., 2015; Blatrix et al., 2018). These biological invasions can also have negative economic impacts on OPSOs, adding to the urgency of addressing this issue (Parrella et al., 2015). Our survey has revealed the presence of invasive Tapinoma ants in OPSOs of all urban areas sampled. It would therefore be essential to allocate more resources to government services dedicated to biological invasion issues. Awareness-raising among national and European authorities, coupled with support for OPSOs staff through training and funding, would be necessary for effective management of these invasion routes (Cronin et al., 2017).
Conclusion
Overall, we reveal a pervasive and complex role of the ornamental plant trade (most probably of trees requiring soil transport) in the human-mediated continental and regional spread of three invasive ants of the Tapinoma nigerrimum complex in western Europe. Using molecular markers, we linked Tapinoma ants found in OPSOs to regions supplying ornamental plants, and most strikingly, showed that there are multiple source populations being introduced into OPSOs. However, our results also highlight that the presence of invasive species in OPSOs does not always translate into the invasion of the surrounding region. Indeed, although we documented high propagule pressure of Tapinoma magnum in OPSOs in all studied French regions, in Montpellier, where other species of the Tapinoma nigerrimum complex occur natively, this invasive species was rare outside OPSOs. In contrast, outside the Mediterranean regions, where no Tapinoma of the nigerrimum complex occur natively, invasive T. magnum populations were found outside OPSOs. This research enhances our understanding of a significant invasion pathway for ants and other invertebrates and stresses on the importance of collaborating with OPSOs to prevent future introductions and limit ongoing ones.
Appendices
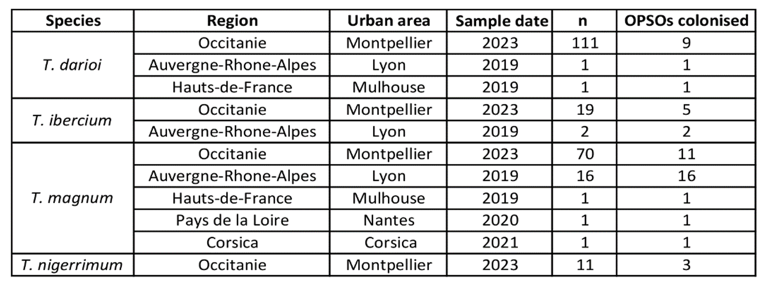
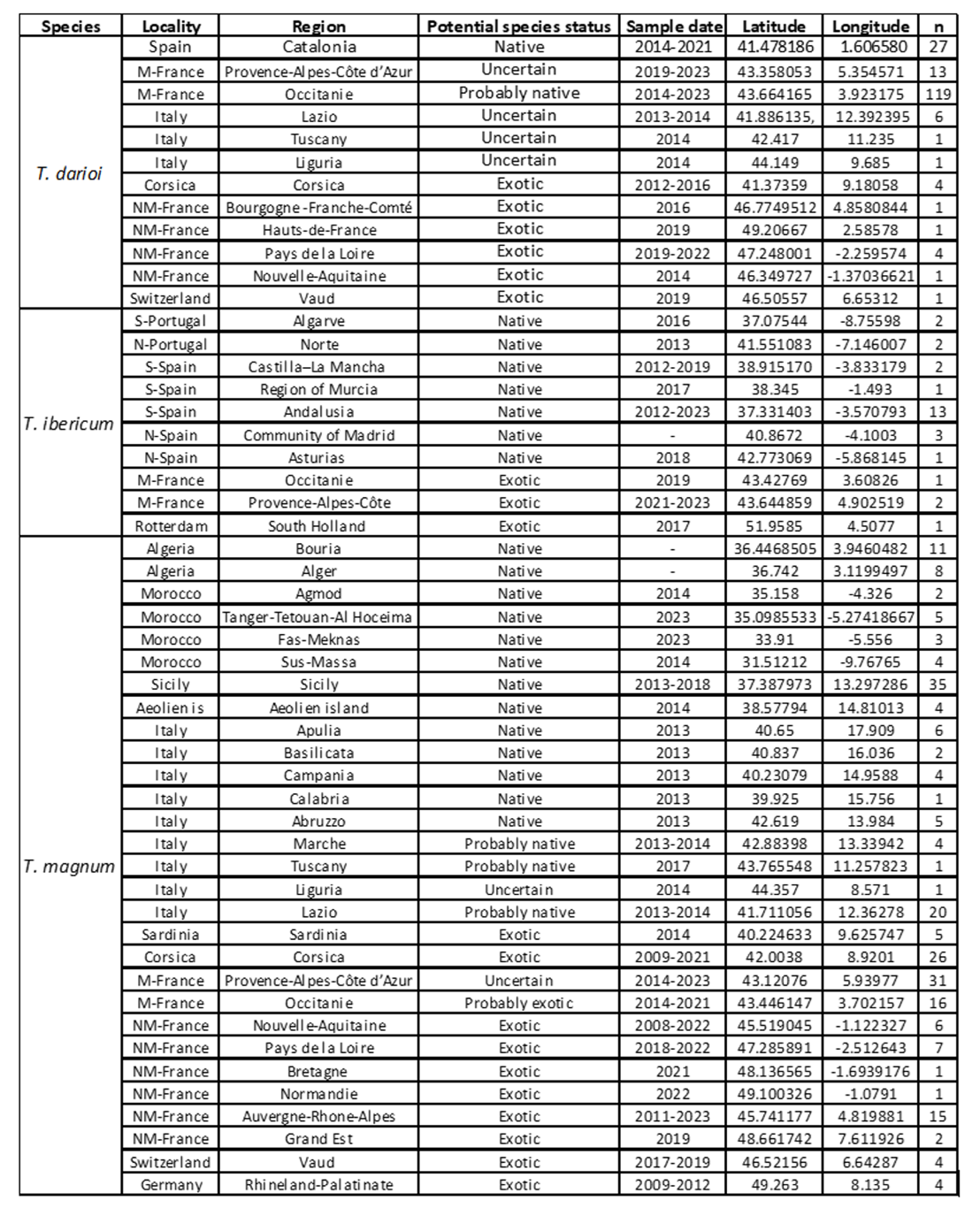
Appendix 1 – Tapinoma samples collected in (A) French OPSOs and (B) Western Europe and North Africa localities.
(A) OPSOs
(B) Localities
Appendix 2 – Diagram of the two-step structure analysis conducted individually for the three Tapinoma species, along with the corresponding figure numbers in the results section. Each vertical line represents a hypothetical individual collected in a French OPSO (blue) or in localities (beige).
Appendix 3 – Ant species of Montpellier OPSOs depending sampling perimeter
Acknowledgements
The authors thank all OPSOs staff for permitting sampling in their stores, Clément Gouraud for providing Tapinoma samples from northwestern France, and Asma Labbaci for providing Tapinoma samples from Algeria. They also thank Pascaline Chiffet-Belle for supervising Asma Labbaci’s laboratory work. They thank the Zoology Collections of the Université Claude Bernard Lyon 1 (UBLZ) for funding the project and ensuring the conservation of all specimens and DNA used in the study. They also acknowledge support from the French National Research Agency (ANR) through the LABEX IMU (ANR-10-LABX-0088) of Université de Lyon, within the Investissements d’Avenir program (ANR-11-IDEX-0007), as well as from the City of Montpellier and Montpellier Méditerranée Metropolis. They warmly thank Bernhard Seifert at the Senckenberg Museum for Natural Sciences in Goerlitz for his support and for sharing important biological material. They are also grateful to Cyril Berquier at the Office de l’Environnement de la Corse for sending numerous specimens from the island. They thank Massimiliano Centorame, formerly at La Sapienza University in Rome, for providing all Italian samples, based on the original sampling by Dario D’Eustacchio. Lastly, they extend their gratitude to the many interns and students who have contributed to ant collections and genetic analyses over the years. Preprint version 2 of this article has been peer-reviewed and recommended by Peer Community In Ecology (https://doi.org/10.24072/pci.ecology.100741; Phillips, 2025).
Funding
The authors are funded by Montpellier Méditerranée Metropolis and the City of Montpellier. B. Kaufmann, A. Dumet and J.M.W. Gippet were supported by the French National Research Agency (ANR) through the LABEX IMU (ANR-10-LABX-0088) of Université de Lyon.
Conflict of interest disclosure
The authors declare that they comply with the PCI rule of having no financial conflicts of interest in relation to the content of the article.
Data, scripts, code, and supplementary information availability
Data are available online: https://doi.org/10.23708/V2AMCA (Destour et al. 2024b).


 CC-BY 4.0
CC-BY 4.0