Introduction
Biological aging is accompanied by structural and neurophysiological changes in the central nervous system (Bishop et al., 2010), as well as a potential deterioration of cognitive functions (Andrews-Hanna et al., 2007). In particular, motor memory processes can be severely impacted, affecting the control and (re)learning of daily tasks (Sawaki et al., 2003; Seidler et al., 2010; Roig et al., 2014) and consequently the autonomy of the person. Inhibitory control is known to play a major role in motor memory programs (Hummel et al., 2002; Sauseng et al., 2013). Also, age-related declines in memory, learning, and attention impair the cognitive resources needed for inhibitory control, leading to increased distractibility, difficulty in suppressing irrelevant information, and reduced behavioral flexibility (Hasher & Zacks, 1988; Gazzaley & D’Esposito, 2007). Preventing the decline of inhibitory control throughout life could be a key strategy for addressing the critical societal challenge of avoiding predictable diseases in young and middle-aged adults and preserving autonomy in older adults.
A specific deficit in inhibitory responses has been observed in older adults compared to youngers adults (Bedard et al., 2002; Coxon et al., 2012). The Go-NoGo (GNG) task and the Stop-Signal Task (SST) are widely employed in research to assess motor impulse control and reactive inhibition, respectively (Cai et al., 2011; Kenemans, 2015; Verbruggen et al., 2019). Overall, performance at the GNG task and SST tends to decline with aging (Kramer et al., 1994; Andrés et al., 2008; Bloemendaal et al., 2016). An age-related reduction of activation in frontal and subcortical areas (Kramer et al., 1994; Coxon et al., 2016) and lower GABA+ level in pre-SMA (Hermans et al., 2018) may explain the deterioration of the behavioral markers of inhibition. However, some inconsistencies exist in the literature regarding the effect of aging on inhibition. For example, some studies found an age-related deficit in SST but not in GNG task (Smittenaar et al., 2015; Kleerekooper et al., 2016; Hermans et al., 2018, 2019). Furthermore, studies using different tasks to measure other forms of inhibition (e.g., Stroop, Flanker tasks) did not support this age-related deficit (e.g., Sebastian et al., 2013), suggesting that different types of inhibition may be differentially affected by aging.
One limitation of previous studies on age-related changes in behavioral inhibition is the use of discrete age groups rather than treating age as a continuous variable. Besides, the age groups are not systematically the same between studies, making it difficult to compare the results. Another limitation is the age range of the older adults, mainly between 60 and 75 years old, even though life expectancy keeps increasing due to better quality of life and advances in health sciences. Therefore, it becomes necessary to include i) a continuum of age, instead of age stratification, and ii) very old individuals who show an acceleration in the alteration of functional cognitive abilities (Van Hooren et al., 2007; Kafri et al., 2019).
In addition to age, the level of physical activity and sedentary behavior may influence the age-related deficit in inhibitory control. According to the World Health Organization, physical activity is any bodily movement produced by skeletal muscles that requires energy expenditure, while sedentary behavior is any period of low-energy expenditure when awake such as sitting, reclining or lying. Sedentary behavior should not be confused with physical inactivity, which is defined as a low level of physical activity, i.e., fewer than the global recommendations of 7500 steps per day for young and middle-aged individuals and 6000 steps per day for old and very old adults (Tudor-Locke et al., 2011a; Tudor-Locke et al., 2011b). As expected, reducing sedentary time by increasing physical activity likely improves cognitive functions (Feter et al., 2024) and overall health (Thyfault et al., 2015). Interestingly, a single session of physical activity (Kao et al., 2023) and a 12-week program of physical activity (Remiszewski et al., 2025) had positive effects on inhibitory control in children with obesity and young adults, respectively. While physical activity and sedentary behavior are essential markers of performance in motor control and cognitive capacities (Falck et al., 2017), they have never been taken into account within the same study across the lifespan when studying behavioral inhibition so far. Therefore, it is essential to consider the decline in cognitive and motor functions in relation to the individual’s level of physical activity and sedentary behavior, whatever the age. Especially, it is crucial to determine the extent to which sedentary behavior alters such functions despite reaching the international recommendations of physical activity (Panahi & Tremblay, 2018).
In this study, we aimed to investigate how age, physical activity, and sedentary behavior influence inhibitory control. We recruited 78 participants (40 women), aged 18 to 88 years old. We measured motor impulse control and reactive inhibition using GNG and SST, respectively. Then, we recorded the level of physical activity (from light to very vigorous) and sedentary behavior using an accelerometer during four consecutive days. We hypothesized that the levels of physical activity and sedentary behavior, in addition to age, would predict variations of inhibitory control across the lifespan.
Results and Discussion
The distribution of the main variables is presented in Figure 1 (the main outcomes are detailed in Supplementary Information – Tables S2 to S11). We estimated motor impulse control with the percentage of NoGo commission errors (i.e., the percentage of key presses in NoGo trials during the GNG task). A greater number of such errors indicates an inability to withhold a potential response. We also reported the reaction time (RT) of correct Go trials in the GNG task, a marker of the ability to respond quickly to a signal. We evaluated reactive inhibition with the Stop-Signal Reaction Time (SSRT) during SST. SSRT is a relevant estimate of the time needed to abord an already-initiated action (Meyer & Bucci, 2016; Verbruggen et al., 2019). The shorter the SSRT, the better the reactive inhibitory control (Aron et al., 2004). The context-independence assumption has been checked. The RT of unsuccessful trials (468 ±105 ms) was significantly lower than the mean RT on go trials (528 ±130 ms, t=-13.89, p<0.001, Cohen’s d=-1.57), supporting that SSRT can be estimated in our sample (Verbruggen et al., 2019).
Seventy-eight individuals (40 women), aged 18 to 88 years old, participated in this study. Seventeen participants (9 women) were aged between 65 and 80 years old, and fourteen (7 women) were above 80 years old. We conducted multiple regression analyses to examine the predictive relationship between age, physical activity, and sedentary behavior on motor impulse control and reactive inhibitory control. This approach allowed us to determine the unique contribution of each predictor while controlling for potential confounding effects.
In addition, to ensure that our sample aligned with findings from the literature, we conducted additional analyses comparing age groups using ANOVAs, without accounting for physical activity and sedentary behavior. These latter comparisons are presented in Supplementary Information (Tables S2 to S5).
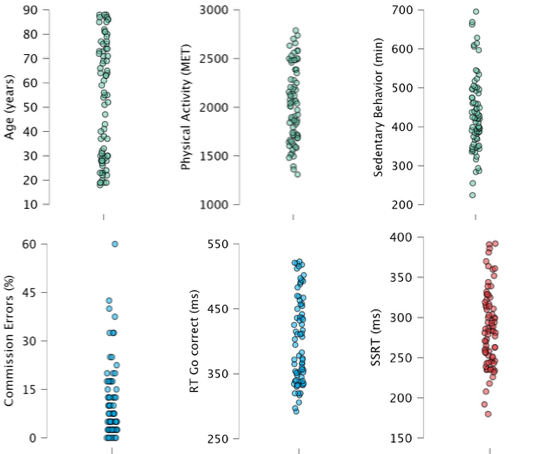
Figure 1 - Distribution of the main variables. Seventy-eight participants were included. The level of physical activity per day was estimated in METs (Metabolic Equivalent of Task) with the formula: PA = Nb min Light Int. * 3 + Nb min Moderate Int.*5 + Nb min Vigorous Int.*7 + Nb min Very Vigorous Int.*9. With PA=Physical Activity, Nb min=number of minutes and Int.=Intensity. For more details about the formula and the selection of the coefficients, see Section Material and Methods. The time per day spent in sedentary behavior was measured in minutes. For the GNG task, we measured the percentage of commission errors (% NoGo incorrect) and the reaction time for correct Go trials (RT Go correct) in milliseconds. For the SSRT task, we measured the Stop Signal Reaction Time (SSRT) in milliseconds.
Motor impulse control
With a multiple regression, we first determined whether our 3 regressors (age, physical activity and sedentary behavior) explained the variability of the percentage of commission errors in the GNG task. The Durbin–Watson test yielded a statistic of 1.71, indicating no significant autocorrelation in the residuals of the regression model. All predictors had VIF values between 1.02 and 1.19, suggesting an absence of multicollinearity. The ANOVA showed that our model did not better predict the percentage of commission errors than the mean model (F(3,74)=0.162, p<0.921, with adjusted R2 of 0.034). This means that none of the 3 regressors could explain the variability of %commission errors in our sample (Figure 2).
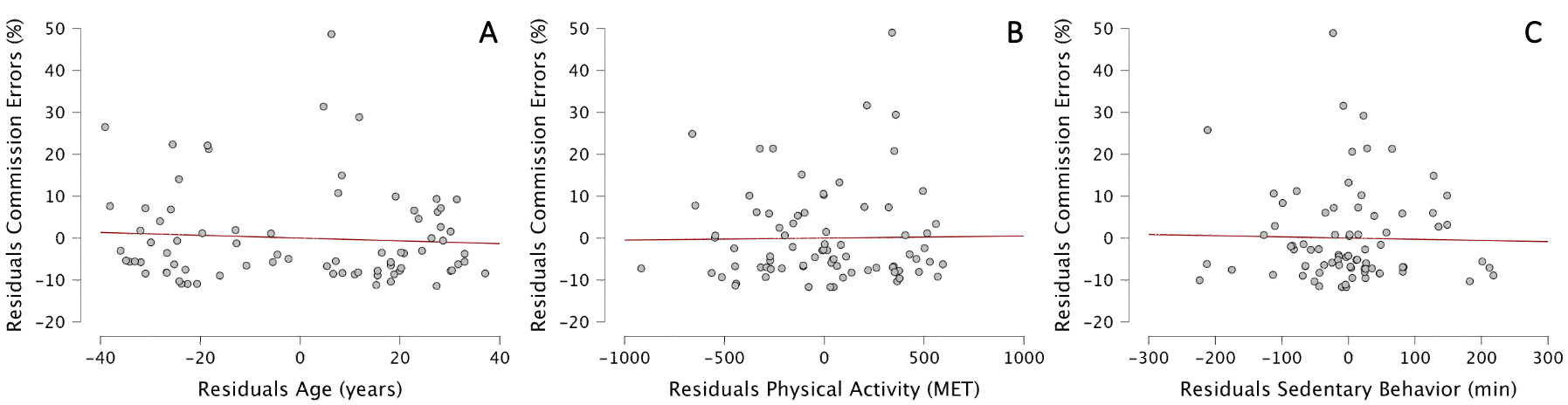
Figure 2 - Partial regression plots between residuals of %commission errors in GNG task and residuals of Age (A), Physical Activity (B), and Sedentary behavior (C).
However, we found an influence of age on RT of correct Go trials in the GNG task. The Durbin–Watson test yielded a statistic of 1.85, indicating no significant autocorrelation in the residuals of the regression model. All predictors had VIF values between 1.02 and 1.19, suggesting an absence of multicollinearity. The ANOVA showed that our model was significantly better at predicting the RT of correct Go trials than the mean model and was a significantly better fit to the data than the mean model (F(3,74)=41.24, p<0.001, with adjusted R2 of 0.611). Our model explained 61.1% of the variability of correct Go trials RT, compared to the mean model.
In details, age, but not physical activity nor sedentary behavior, positively predicted RT of correct Go trials [b=2.47; t=10.99, p<0.001]. For each additional year, RT of correct Go trials increased by 2.47 ms. The standardized coefficient of Age is 0.791 (Figure 3).
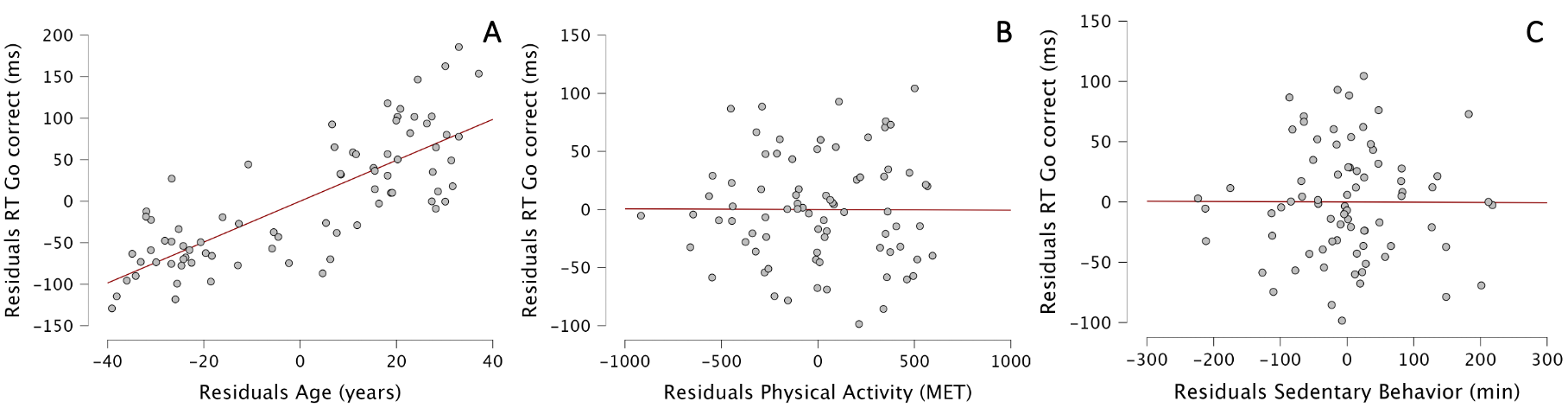
Figure 3 - Partial regression plots between residuals of RT of correct Go trials and residuals of Age (A), Physical Activity (B), and Sedentary behavior (C).
Reactive inhibition
With a multiple regression, we then determined whether our 3 regressors (age, physical activity and sedentary behavior) explained the variability of SSRT. The Durbin–Watson test yielded a statistic of 1.93, indicating no significant autocorrelation in the residuals of the regression model. All predictors had VIF values between 1.02 and 1.19, suggesting an absence of multicollinearity. The ANOVA showed that our model was significantly better at predicting SSRT than the mean model and was a significantly better fit to the data than the mean model (F(3,74)=18.23, p<0.001, with adjusted R2 of 0.402). Our model explained 40.2% of the variability of SSRT, compared to the mean model.
In details, both age and sedentary behavior were included in the model, meaning that physical activity could not predict SSRT. Interestingly, age [b=0.899; t=5.15, p<0.001] and sedentary behavior [b=0.229; t=4.90, p<0.001] positively predicted SSRT. For each additional year, SSRT increased by 0.899 ms, and for each additional hour of sedentary behavior per day, SSRT increased by 13.74 ms. In other words, one additional hour of sedentary behavior per day would alter reactive inhibition as much as 15.3 years of life. A very old person with low sedentary behavior would be more likely to stop an action than a younger person with higher sedentary behavior. The standardized coefficients of age and sedentary behavior are 0.459 and 0.467, respectively, showing the equal importance of these two factors to explain SSRT (Figure 4).
The international recommendations consider not to exceed 6 to 8 hours of sitting/lying down during daytime (Dempsey et al., 2020). To further explore our results, we split our sample into 2 groups (Sedentary Behavior, n=37 vs. Non-Sedentary Behavior, n=41) using 7 hours per day of sedentary behavior as the cut-off. We then performed an ANCOVA with SSRT as the dependent variable, Group as the main factor and Age as the covariate. We found a main effect of Group (F1,75=10.19, p=0.002, η2p=0.12; Non-Sedentary Behavior group = 271 ±40 ms < Sedentary Behavior group = 300 ±48 ms) and an effect of Age (F1,75=23.84, p<0.001, η2p=0.24; see Figure 5). This finding indicates that sedentary behavior alters reactive inhibition across the lifespan, even if the participants reach the international recommendations.
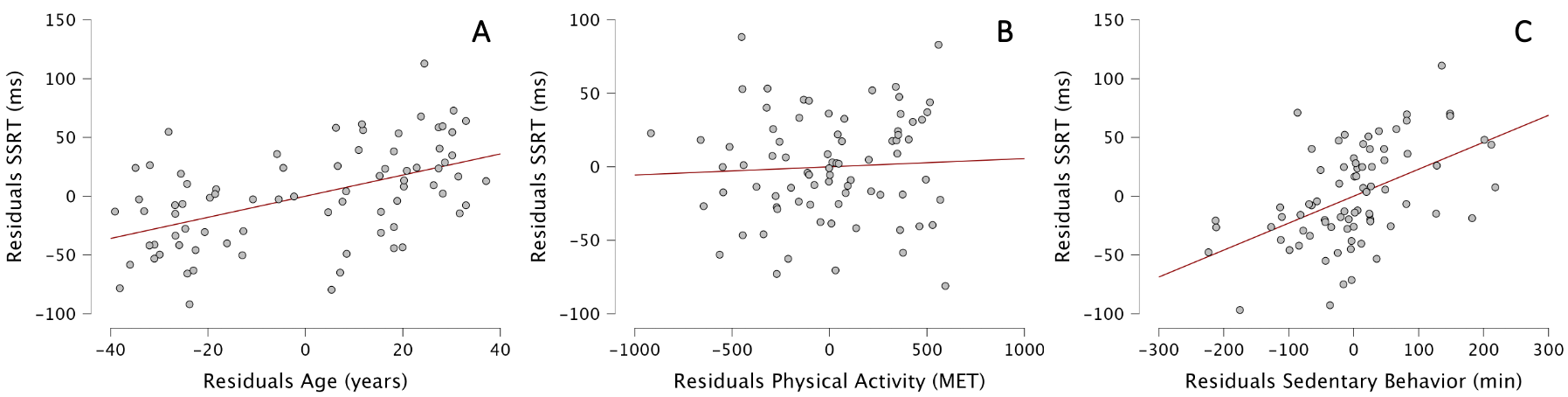
Figure 4 - Partial regression plots between residuals of SSRT and residuals of Age (A), Physical Activity (B), and Sedentary behavior (C).
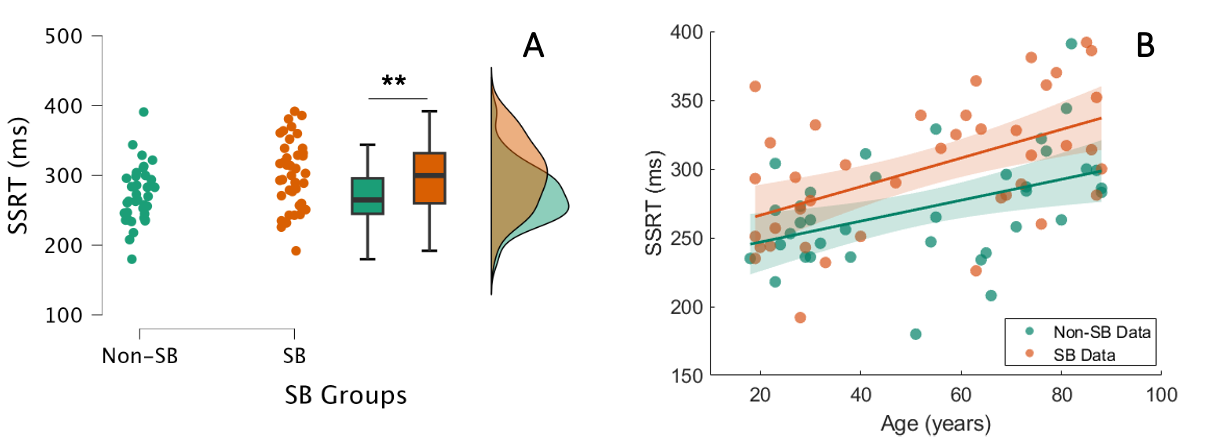
Figure 5 - A. Reactive inhibition and sedentary behavior. The mean SSRT of individuals with non-sedentary behavior (Non-SB, 271 ±40 ms) is significantly lower than that of individuals with sedentary behavior (SB, 300 ±49 ms). B. Correlation between age and SSRT. Both groups presented a relationship between age and SSRT (Non-SB group: r=0.442, p=0.006; SB group: r=0.528, p<0.001). The older the participant, the longer the reactive inhibition, regardless of the group.
Main discussion
As expected, age partially predicted motor impulse control and reactive inhibitory behavior (Bedard et al., 2002). In general, the older adults take longer 1) to respond to a Go signal when NoGo signals are present within the blocks and 2) to prevent from moving when a Stop signal urgently asks to refrain from the planned action. Notably, we found no effect of age on the percentage of commission errors in the GNG task, another marker of motor impulse control.
Interestingly, the main result of this study was that sedentary behavior, but not physical activity, predicted the change of reactive inhibition to a similar level as age. One hour of additional sedentary behavior lengthens reactive inhibition as much as 15.3 years of life. This suggests that an older person with lower sedentary behavior may perform similarly to, or even better than, a younger person with high sedentary behavior. Surprisingly, physical activity may not predict reactive inhibition and therefore does not seem to mitigate the negative effects of sedentary behavior. This result goes against previous findings showing that adding a single session or a program of several sessions of physical activity improved inhibitory control (Kao et al., 2023; Remiszewski et al., 2025). However, previous studies did not measure both physical activity and sedentary behavior to test their specific influence on inhibitory control. Increasing the time in physical activity automatically decreases the time in sedentary behavior. Therefore, the positive effects on inhibitory control could also be interpreted as a consequence of the reduction in sedentary behavior.
In our sample, all participants met these international recommendations (Figure 6). Here, we expressed international recommendations for physical activity in step count, rather than METs, as they were more easily comparable when using accelerometers. In our sample, physical activity in METs and step count were significantly correlated (for young and middle-aged participants: Pearson’s r=0.764, p<0.001; for old and very old participants: Pearson’s r=0.839, p<0.001). Therefore, even individuals who meet physical activity guidelines may still experience negative effects if they engage in prolonged sedentary behavior (Panahi and Tremblay, 2018). Our original findings highlight the detrimental effect of sedentary behavior across the lifespan on cognitive functions, such as inhibitory control, regardless of physical activity.
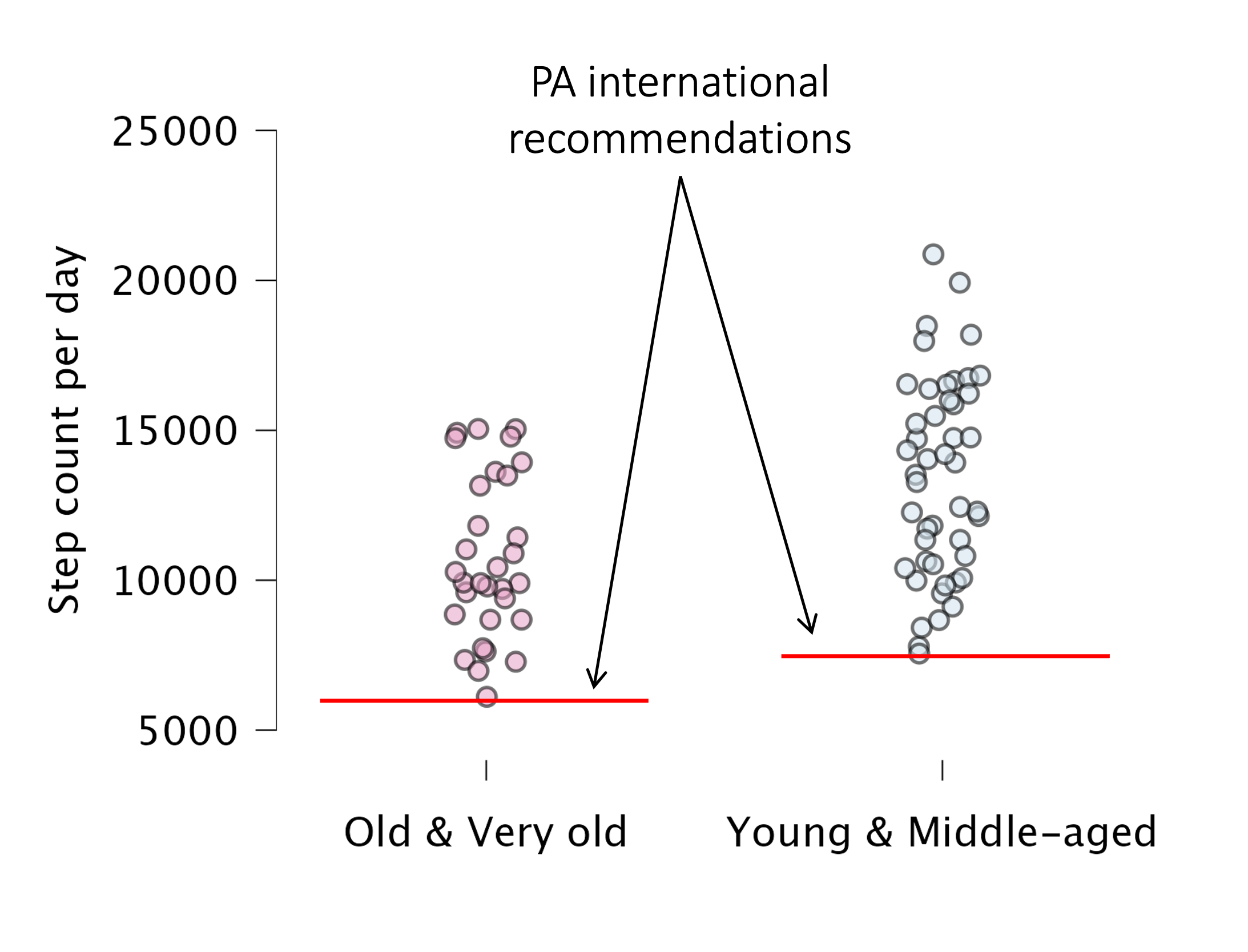
Figure 6 - International recommendations of physical activity (PA) in step count. The threshold for Old-Very old and Young-Middle-aged persons is set at 6000 and 7500 steps per day, respectively. In our sample, all participants met this criterion.
Such effects, i.e. absence of physical activity influence on inhibitory processes and specific influence of sedentary behavior on reactive inhibition, may be explained by specific brain activation networks. Van Belle et al. (2014) demonstrated common and exclusive networks during motor impulse control and reactive inhibition. The connectivities dlPFC/ACC, vlPFC/pre-SMA/IPL and right vlPFC/right IPL are commonly activated during both types of inhibition. The superior parietal gyrus, the angular gyrus and the superior occipital gyrus are exclusively activated during motor impulse control, whereas the connectivities right dlPFC/right IPL, right Frontal/bilateral Temporal and right vlPFC/preSMA are exclusively activated during reactive inhibition.
The right Frontal/bilateral Temporal connectivity, and especially the temporal lobe, seem to be a key area to explain our results. Indeed, Siddarth et al. (2018) found that the total thickness of medial temporal lobe in adults aged 45 to 75 years old was inversely correlated with hours of sitting per day, but not with the level of physical activity.
Besides, the temporal lobe is associated with both impulsivity and inhibitory control. In particular, the neural networks between ACC, IFG and Inferior temporal gyrus is of importance to modulate impulsivity (Li & Kong, 2017). This is demonstrated by higher motor impulsivity, i.e., lower inhibitory control, in patients with temporal lobe epilepsy (De Oliveira et al., 2011).
Alongside, healthy and pathological individuals with impulsivity traits present altered inhibitory control in general, supported by longer SSRT at the stop-signal task (Schachar et al., 1993; Logan et al., 1997). Therefore, increasing sedentary time may reduce the functional activity of the temporal lobe and, by cascade, of the connected neural network, resulting in impaired inhibitory control, particularly reactive inhibition. Similar to cognitive deficits, high levels of sedentary behavior have been associated with higher risks of cardiovascular diseases, metabolic disorders or cancers (Saunders et al., 2020), independently of moderate-to-vigorous physical activity (Panahi and Tremblay, 2018). This indicates again that, in this sample, physical activity did not appear to mitigate the association between sedentary behavior and inhibitory control.
Main message
While the current international recommendations focus on increasing the level of physical activity of the general population and even more of the older population, our results demonstrate that the reduction of sedentary behavior is of higher importance to maintain cognitivo-motor abilities, such as reactive inhibition processes. Even though one follows the recommendations for the level of physical activity, i.e., 6000 and 7500 steps per day, such amount would not be sufficient to suppress the harms of increased sedentary behavior at home and at work (Thyfault et al., 2015; Ekelund et al., 2016; Panahi & Tremblay, 2018), as often observed in our modern society.
Perspectives
Future studies should focus on the reduction of sedentary behavior and its impact on cognitivo-motor functions, especially in the elderly population. Indeed, such intervention is considered more acceptable by older adults than the increase of the level of physical activity, which they perceive as less compatible with their age (Devereux-Fitzgerald et al., 2016; McGowan et al., 2018). To support our findings, it would be relevant to test whether reducing the time in sedentary behavior would improve reactive inhibition, i.e., reduce SSRT. Besides, non-invasive brain stimulation would be a good candidate to modulate the activity of the temporal lobe in patients with impaired inhibitory control in order to test whether this is a key area in reactive inhibition associated with sedentary behavior.
Limitation
While our sample of participants covers all ages (but with a smaller proportion around 50 years old, see Figure 1A), the level of physical activity and sedentary behavior, especially of the older participants, may not reflect the general population. A first limitation might be the level of physical activity of our sample. The recruited participants might have a higher level of physical activity than that of the general population, as they all met the international recommendations. This could hide any potential effect of this parameter on inhibitory behavior. Similarly, the time spent in sedentary behavior did not significantly differ between the age groups when using stratification (see Table S7 in Supplementary Information), whereas it usually tends to increase with age (Harvey et al., 2015). Although accelerometers are not primarily designed to assess sedentary behavior, there is no gold standard to date and accelerometers allow an objective quantification of sedentary behavior compared to questionnaires. Nonetheless, despite the homogeneity of sedentary behavior across age in our sample, we found a strong effect of sedentary behavior on reactive inhibition. This further supports the main conclusion that sedentary behavior is a main driver of inhibitory control decline. Finally, future research could benefit from the inclusion of other variables, such as socio-economic status, body mass index, muscle atrophy, or obesity.
Material and Methods
Participants
To observe any effect using a multiple regression (3 regressors – age, physical activity and sedentary behavior) with an anticipated moderate effect size (0.15), a power of 0.8, and a probability level of 5%, we estimated that at least 76 participants would be needed, using G* Power (version 3.1.9.4., (Faul et al., 2007). We recruited 78 participants (40 women) aged 18 to 88 years old, across two sites (Dijon and St-Etienne, France), via email, through postings at the universities, and by word of mouth. All procedures were ethically approved (Dijon: IRB00012476-2022-05-04-172; St-Etienne: CPP Nord-Ouest 1 #21.00901.000003). The study complied with the standards set by the Declaration of Helsinki (Version, 2013; excluding pre-registration in a database). Informed consent was obtained from all participants.
Measure of physical activity and sedentary behavior
To quantify the level of physical activity and sedentary behavior, the participants wore an accelerometer (wGT3X-BT; ActiGraph, Pensacola, FL) at their wrist for 4 consecutive days including two weekdays and two days of the weekend. For those who wore the watch at night, we did not take into account the sleeping period, using time filter within Actilife version 6 software (ActiGraph), as sedentary behavior (in minutes) was identified as any period of low-energy expenditure when awake such as sitting, reclining or lying, including daylight naps. We estimated the level of physical activity (in MET) with the sum of the weighted duration of each intensity (light, moderate, vigorous and very vigorous intensities). The coefficients were 3, 5, 7 and 9 for light, moderate, vigorous and very vigorous intensities, respectively (Liu et al., 2017; Mendes et al., 2018).
Motor impulse control
The GNG task was used to probe motor impulse control. Custom-made software presented green or red circles (Figure 7) on a 17-inch flat screen. The participants had to press the space bar of the keyboard, with the right hand, as fast as possible in response to the Go trials (green circles) and to not respond to NoGo trials (red circles). Since the participants knew in advance whether to move (press the space bar) or not, this protocol design captured the anticipatory suppression of action, a mechanism involved in motor impulse control.
After a familiarization block (8 Go and 2 NoGo trials, randomized), the participants performed 4 blocks of 50 trials, with a proportion of 80% of Go trials (20% of NoGo trials) and with 45-sec rest between blocks. In total, there were 200 trials with 160 Go and 40 NoGo trials. The intervals between signals varied from 1000 ms to 1500 ms, with a maximal appearance duration of 2000 ms. We measured i) the percentage of commission errors, i.e., the number of key presses during NoGo trials divided by the total number of NoGo trials, and ii) the reaction time of correct Go trials.
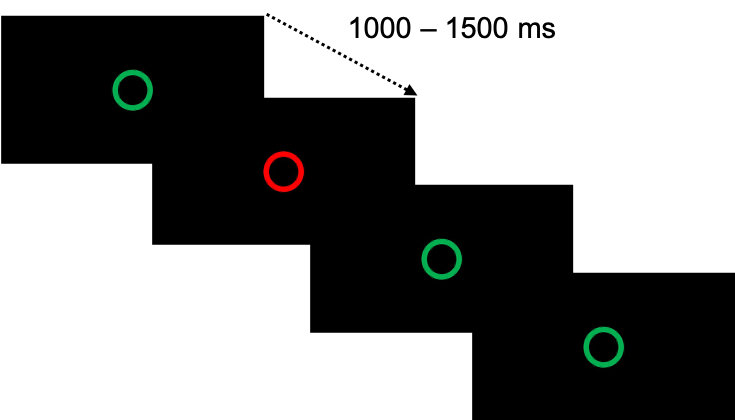
Figure 7 - Signals for the Go/NoGo task. The participants had to press the space bar of the keyboard as fast as possible in response to the Go trials (green circles) and to not respond in NoGo trials (red circles). The intervals between signals varied from 1000 ms to 1500 ms, with a maximal appearance duration of 2000 ms.
Reactive inhibition
The Stop-Signal Task was used to probe reactive inhibition. We used the online version developed by F. Verbruggen https://github.com/fredvbrug/STOP-IT/tree/master/jsPsych_version.
The participants had to press the left or right arrow of the keyboard, with the index or the middle finger of the right hand respectively, as fast as possible in response to the Go trials (left or right arrow) and to not respond to Stop trials (when the arrows turned red following a varying stop signal delay; Figure 8). There were 25% of Stop trials. A staircase algorithm was used to ensure an equal number of correct and failed stop trials, i.e., p(inhibit)=0.5. Since the participants were instructed to perform a movement on each trial, with a small proportion of Stop signals after the Go signal, the protocol design captured their ability to cancel ongoing motor actions, reflecting a reactive mechanism.
After a familiarization block of 64 trials, the participants performed 4 experimental blocks of 64 trials each, with 15-sec rest between blocks. In total, there were 256 signals, with 192 Go trials and 64 Stop trials. Between each block, each participant received feedback on their mean RT, and their proportion to correctly inhibit their response. This helped the experimenter to ensure that RT of correct Go trials did not increase between blocks. If this was the case, the experimenter recalled the instruction to respond as fast as possible.
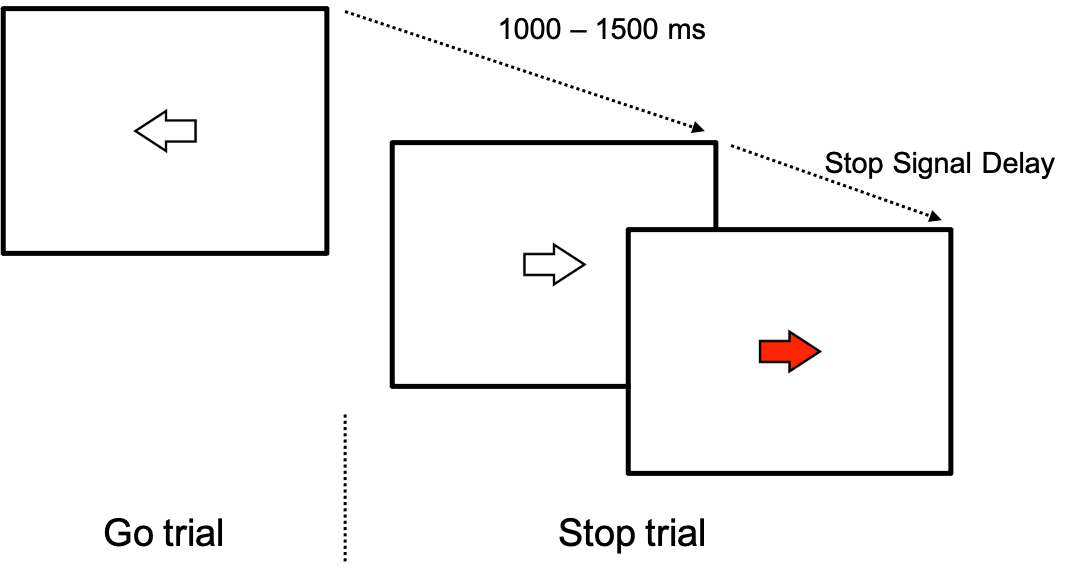
Figure 8 - Stop-Signal Task design. The participants had to press the left or right arrow of the keyboard, with the index or the middle finger of the right hand respectively, as fast as possible in response to the Go trials (left or right arrow) and to not respond in Stop trials (when the arrows turned red following a variable stop signal delay).
Statistical analysis
Multiple regressions were used to predict motor impulse control and reactive inhibition from age, sedentary behavior and physical activity. We used a classical approach to determine the model that best predicts the dependent variable (% commission errors and RT for correct Go trials for motor impulse control and SSRT for reactive inhibition). For each participant, we excluded RT trials that were below or above the mean ± 3 standard deviations. We tested for independence of the variables (Durbin-Watson), homoscedasticity (visual inspection of Q-Q plots) and absence of multicollinearity (using VIF scores, Hair et al., 2009). Then, by means of an ANOVA, we determined whether the model with the three regressors (age, sedentary behavior and physical activity) better predicted the dependent variable than the mean model. When significant, we detailed the influence of each predictor. In exploratory analyses, we split our sample into 2 groups, based on the international recommendations for the level of sedentary behavior or physical activity. The first exploratory analysis was an ANCOVA with SSRT as the dependent variable, Sedentary Group as the main factor and Age as the covariate. We used 7 hours per day of sedentary behavior as the cut-off to identify our groups. The second exploratory analysis was performed to visualize the distribution of our sample based on the global recommendations of 7500 steps per day for young and middle-aged individuals and 6000 steps per day for old and very old adults. As supplementary analyses, we performed ANOVAs with age groups as a between-subject factor, without accounting for physical activity and sedentary behavior, to ensure that our data aligned with the literature (see Supplementary Information). Alpha level was set at 0.05. We used JASP (Version 0.19.3) for our statistical analysis.
Abbreviations
ACC = Anterior Cingulate Cortex; dlPFC = dorso-lateral PreFrontal Cortex; GNG = Go/NoGo; IFG = Inferior Frontal Gyrus; IPL = Inferior Parietal Lobe; PA = Physical Activity; pre-SMA = pre-Supplementary Motor Area; RT = Reaction Time; SB = Sedentary Behavior; SSRT = Stop-Signal Reaction Time; SST = Stop-Signal Task; vlPFC = ventro-lateral PreFrontal Cortex.
Acknowledgements
We thank Cyril Sirandre for developing the software used for the Go/NoGo task and Frederik Verbruggen for sharing the online version of the Stop Signal Task. Preprint version 4 of this article has been peer-reviewed and recommended by Peer Community In Neuroscience (https://doi.org/10.24072/pci.neuro.100225; Boisgontier, 2025).
Funding
This work was supported by the Institut Universitaire de France (IUF, Paris, France).
Author contribution
Florent Lebon: Conceptualization, Methodology, Formal analysis, Writing - Original Draft, Writing - Review & Editing, Supervision, Project administration; Mathilde Bertrand: Data collection, Formal analysis, Writing - Review & Editing; Boris Genand: Data collection, Formal analysis; France Mourey: Methodology, Writing - Review & Editing; Célia Ruffino: Writing - Review & Editing; Charalambos Papaxanthis: Writing - Review & Editing; Jérémie Gaveau: Writing - Review & Editing; Vianney Rozand: Methodology, Formal analysis, Writing - Original Draft, Writing - Review & Editing, Supervision.
Declaration of interest
Florent Lebon and Jérémie Gaveau currently acts as members of the managing board of PCI Neuroscience and PCI Health and Movement Sciences, respectively. Throughout the entire review process, they remain blind to any decisions or discussions between the managing board of PCI Neuroscience, the recommender and the reviewers. The authors declare they comply with the PCI rule of having no financial conflicts of interest.
Data, scripts, code, and supplementary information availability
Analyzed data and supplementary tables are available online at https://doi.org/10.17605/OSF.IO/EX4Q6 (Lebon, 2025). The Stop-Signal Task was used to probe reactive inhibition. We used the online version developed by F. Verbruggen https://github.com/fredvbrug/STOP-IT/tree/master/jsPsych_version.


 CC-BY 4.0
CC-BY 4.0