Introduction
Motor imagery refers to the mental simulation of an action without any overt movement, but shares overlapping neural substrates with motor execution (Jeannerod, 1994). A key point of debate has been the involvement of the primary motor cortex (M1) during motor imagery. In an elegant study, Persichetti et al. (2020) probed the activation of distinct cortical layers within the hand-knob region of M1, during both actual and imagined movements. Using the vascular space occupancy technique, they achieved sub-millimeter spatial resolution without the vasculature bias of the BOLD signal. Their main finding was that imagined finger movements activated only the superficial layers II/III with mainly cortico-cortical connections to M1, whereas actual finger movements activated both superficial and deeper layers Vb/VI, with descending corticospinal projections. This result offers a compelling explanation for the absence of muscle activity during motor imagery. However, the exclusive activation of superficial layers in M1 during motor imagery appears inconsistent with numerous previous observations in the current literature. The purpose of this opinion letter is to present studies using various methodological approaches that support the idea of neural modulation downstream of pyramidal cells while imagining. These modulations, following motor imagery practice, alongside changes observed within M1 itself (Pascual-Leone et al., 1995), would also explain improvement in motor learning (Ruffino et al., 2017). We first summarize findings based on transcranial magnetic stimulation (TMS) studies demonstrating increased corticospinal excitability. We then discuss evidence of activity at the spinal cord level, despite the absence of muscle contraction.
Activation along the corticospinal tract
Early motor imagery studies reported a subliminal activation in the targeted muscles (Jacobson, 1930), i.e., below the neural threshold needed to elicit an overt action. In his motor simulation theory, Jeannerod considered that a subthreshold motor activation occurred during motor imagery, and that the “leaks” at the periphery would be the result of an incomplete inhibition at the central level (Jeannerod, 1994). Such evidence supports the idea that motor imagery practice leads to broader neural reorganization across the motor system, not just within M1 (Ruffino et al., 2017). Furthermore, animal studies have demonstrated that superficial cortical layers can influence cortical motor output via strong direct connections to layer V corticospinal neurons (Anderson et al., 2010). Unless an inhibitory mechanism blocks these connections, the activation seen in the superficial layers during motor imagery and reported by Persichetti et al. (2020) should also propagate to deeper layers.
In humans, numerous TMS studies have shown an increase of corticospinal excitability during motor imagery (for review, see Grosprêtre et al., 2016). According to Di Lazzaro & Ziemann (2013), the axons of the more superficial pyramidal neurons (P2/P3) are conceivably the most excitable neural elements to low-threshold TMS, due to their proximity to the stimulating coil. These axons also represent the main source of excitatory descending input to layer V pyramidal tract neurons (Anderson et al., 2010). Since TMS preferentially excites the axons of superficial layer cells and motor imagery increases TMS-evoked responses, it is highly plausible that the superficial pyramidal neurons activated while imagining directly excite the deeper layers (Figure 1).
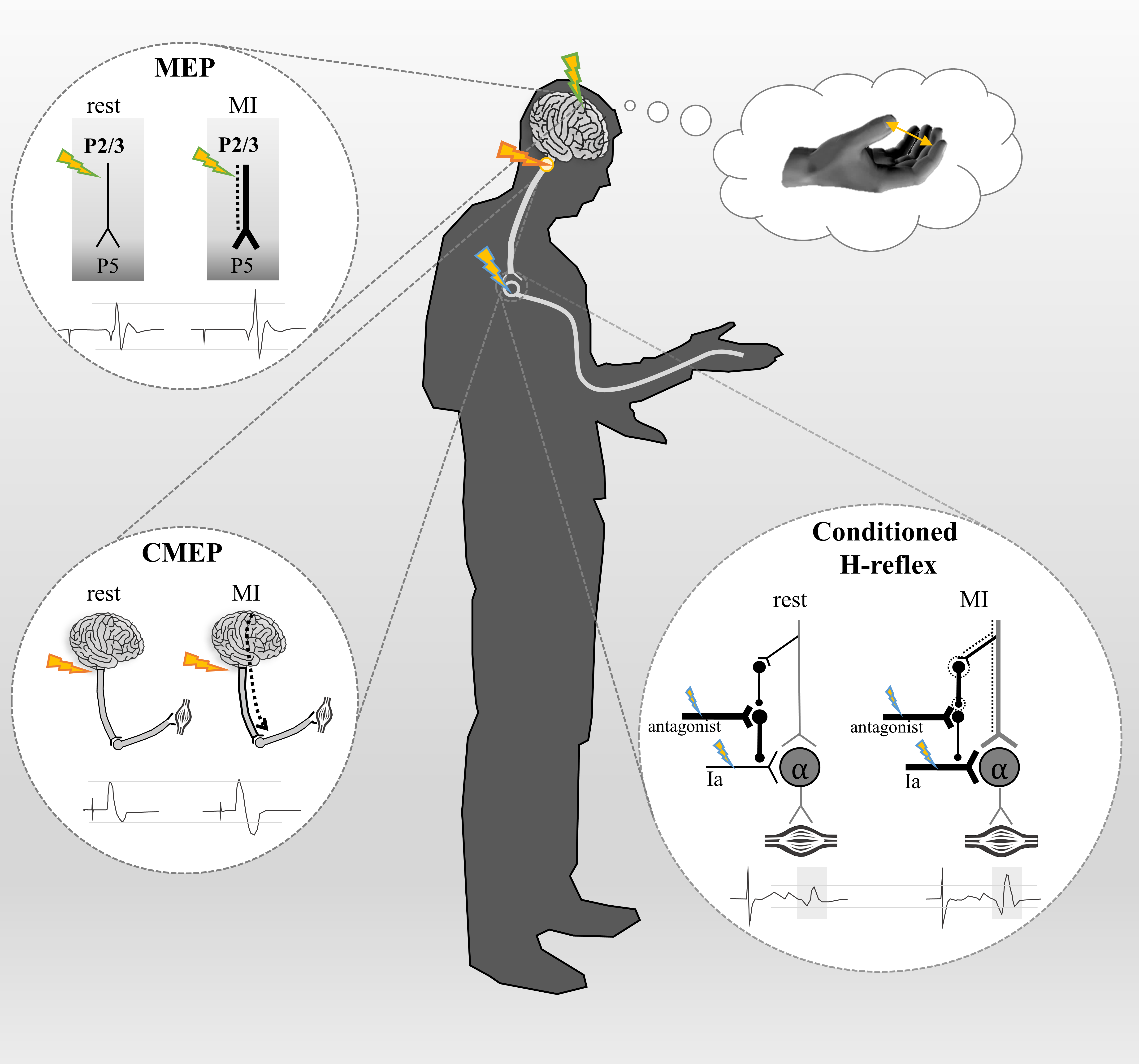
Figure 1 - Schematic illustration of corticospinal pathway activation at supraspinal and spinal levels during imagined movement as probed by different methodological techniques. The corticospinal tract (represented in grey) originates from pyramidal cells in the layer V (P5) of the primary motor cortex to spinal motoneurons, and then to the muscle. Dotted lines represent the subliminal motor output generated during motor imagery (MI). Studies using transcranial magnetic stimulation (TMS) applied over the primary motor cortex (green flashes) have reported an increase of motor-evoked potentials (MEP) amplitude during MI compared to rest. This increase in MEP amplitude is an index of increased corticospinal excitability. Due to their proximity to the TMS coil, the axons of the superficial pyramidal neurons (layers II, III) are the most excitable elements. These neurons are known to have a monosynaptic excitatory connection with layer V. Therefore, if TMS activates superficial layer cells in the primary motor cortex and MI increases MEP amplitude, then it is likely that the layers II,III cells directly excite the deeper layers (layer V) during MI. Electrical stimulation applied at the cervicomedullary junction (orange flashes) elicits cervico-medullary-evoked potentials (CMEP), a direct marker of the cortico-motoneuronal pathway at the spinal level. Grosprêtre et al. (2015) reported an increase in CMEP amplitude during MI compared to rest, suggesting a subliminal activation of the corticospinal tract beyond the pyramidal tract neurons. The Hoffman reflex (H-reflex) which is conditioned by stimulation of the antagonistic nerve (blue flashes) induces D1 presynaptic inhibition of Ia afferents onto alpha motoneurons. Consequently, the conditioned H-reflex amplitude is lower than the unconditioned H-reflex amplitude when measured at rest. However, during MI, the amplitude of the conditioned H-reflex is no longer reduced (Grosprêtre et al., 2015). This suggests that the subliminal output generated during MI is strong enough to activate low-threshold interneurons (black dotted circles), which modulate the state of presynaptic interneurons at the spinal level.
An alternative explanation that aligns with the findings of Persichetti et al. (2020) is the involvement of motor-related cortical areas close to M1 during motor imagery. For example, the dorsal premotor cortex (PMd), which is activated during motor imagery, can directly excite the pyramidal tract neurons via a short‐latency facilitatory PMd‐to‐M1 hand pathway (Groppa et al., 2012). Combined activity from PMd and superficial layers of M1 activities during motor imagery may generate descending volleys in the corticospinal tract when low-threshold TMS pulses are applied. However, this hypothesis remains speculative and needs further investigations. Nonetheless, this hypothetical mechanism cannot fully account for observed increases in neural activity below the pyramidal tract neurons.
Spinal activity
More recently, studies have discovered neural activation below the pyramidal neurons when muscle activity was silent, highlighting the modulation of the spinal circuitry while imagining. For example, Grosprêtre et al. (2015) supported the hypothesis that a subliminal output is generated during motor imagery, using various stimulation techniques. First, they reported an increase of cervico-medullary-evoked potential (CMEP) amplitudes when imagining a muscle contraction, while the H-reflex amplitude remained unchanged (Figure 1). The CMEP provides a direct measure of motoneuronal pool excitability by activating descending axons at the spinal decussation, whereas H-reflex is an index of Ia-alpha synapse excitability. This first finding suggests a subliminal activation of the corticospinal tract during motor imagery, but insufficient to recruit alpha motoneurons. Further, a conditioning technique was used to reveal the activation of low-threshold spinal structures. The H-reflex was conditioned by the stimulation of the antagonist nerve inducing D1 presynaptic inhibition. At rest, this conditioning reduced the H-reflex amplitude, consistent with effective presynaptic inhibition. However, during motor imagery, the inhibitory effect on H-reflex amplitude was no longer observed. This suggests that the subliminal descending volleys generated while imagining may activate low-threshold spinal elements known as primary afferent depolarization interneurons, which in turns suppress presynaptic inhibition (Figure 1). Although we cannot totally rule out the potential contribution of premotor areas to the corticospinal projection (Maier, 2002), there is a strong probability that the pyramidal tract neurons are activated during motor imagery, producing a subliminal motor output that reaches the spinal level.
Conclusion and perspectives
By using the vascular space occupancy technique to capture neural activations across cortical laminae with a sub-millimeter resolution, Persichetti et al. (2020) have provided valuable insights into layer-specific activity in M1 during imagined finger tapping. This work constitutes a clear methodological advance and a promising tool to probe neural networks of motor imagery in humans. Nonetheless, we believe that further methodological considerations are warranted in light of existing literature. For example, when using imaging techniques as in Persichetti et al. (2020), a direct comparison between actual and imagined finger movements would reveal complementary findings. In addition, to further explore this question of M1 activation, non-invasive brain stimulation techniques are relevant tools. One possible approach would be to stimulate, by means of TMS, regions of interest that project, directly or indirectly, to spinal motoneurons (bypassing M1) and to probe whether responses at the spinal level are modulated. The ventral premotor cortex (PMv) is an ideal candidate, since it has strong connections with spinal structures during actual contractions (Braaß et al., 2023), and its central role in motor imagery, with action-specific and goal-related functions (Hanakawa et al., 2003; Hétu et al., 2013; Hardwick et al., 2018). A key methodological challenge, however, would be to stimulate PMv without activating M1, which would require the use of low-intensity TMS pulses.
Another promising direction to investigate the motor system engagement would be to consider the intra- and inter-variability in imagery abilities. Most studies rely on group-level inference statistics to analyze neural modulation during motor imagery. Currently, an emerging literature highlights a continuum of imagery vividness, ranging from aphantasia to hyperphantasia (Zeman, 2024). Identifying neural activation patterns at the individual level would contribute to defining the unique “fingerprints” of motor imagery. This needs to be done with appropriate sample size and adequate statistical analyses (e.g., direct comparisons between conditions, linear mixed models).
Finally, improving the reporting of imagery protocols is essential for reliability and reproducibility, as already emphasized in the context of action observation and motor imagery interventions (Moreno-Verdú et al., 2024). For example, specifying parameters such as the quality and clarity of the imagery instructions, the modality of motor imagery (kinesthetic vs. visual), the intensity of imagined movements, and individual imagery capacity, is of importance.
In conclusion, we argue that the apparent discrepancy between the findings of Persichetti et al. (2020) and those of previous literature highlights a gap in our understanding that merits further investigation.
Acknowledgement
Preprint version 2 of this article has been peer-reviewed and recommended by Peer Community In Health & Movement Sciences (https://doi.org/10.24072/pci.healthmovsci.100200; Hardwick, 2025).
Funding
This work was supported by the French “Investissements d’Avenir” program, project ISITE-BFC (contract ANR-15-IDEX-0003).
Author contributions
Florent Lebon and Cécilia Neige wrote the correspondence, and contributed equally to this work.
Conflict of interest disclosure
Florent Lebon acts as a member of the managing board of PCI Neuroscience. Throughout the entire review process, he remained blind to any decisions or discussions between the managing board of PCI Health and Movement Sciences, the recommender and the reviewers. The authors declare they comply with the PCI rule of having no financial conflicts of interest.


 CC-BY 4.0
CC-BY 4.0